Off-the-shelf CAR-T cell therapy against host immune rejection
In recent years, CAR-T cell therapy has become a promising cancer treatment. However, due to the high cost of CAR-T therapy and the differences in patients' own lymphocytes, only a small number of patients benefit from CAR-T cell therapy. In order to reduce production costs and ensure high-quality treatment results, researchers began to develop universal cell therapies, that is, off-the-shelf cells. It is expected that lymphocytes from healthy people can be used for allogeneic reinfusion therapy, which can reduce costs and production cycles while ensuring product quality, so that patients can receive more immediate and effective treatment. However, allogeneic reinfusion faces two major problems: graft-versus-host disease (GvHD) and host-versus-graft reaction (HvGR). In order to solve these problems, Maksim Mamonkin's research group at Baylor College of Medicine in Houston, Texas, USA designed an allogeneic immune defense receptor ADR through genetic engineering that can effectively resist host immune rejection. Researchers found that 4-1BB is a cell receptor whose expression is temporarily up-regulated after lymphocytes (CD4+, CD8+, NK) are activated. They constructed the recognition region derived from 4-1BBL through the intra-sibling signal CD3ζ in the transmembrane region, allowing it to recognize and resist activated T cells and NK cells, while retaining resting immune cells .
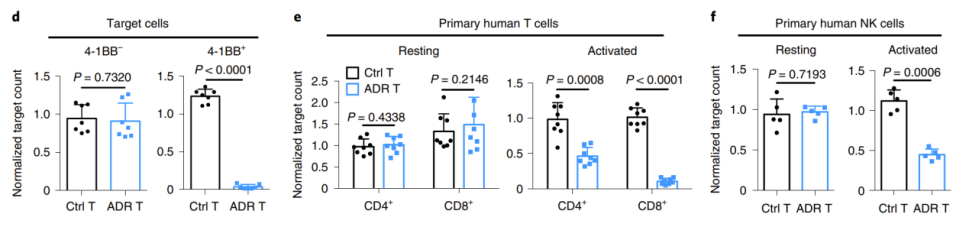
First, the researchers co-cultured ADR-transduced T cells with human primary resting T cells, NK cells, and activated T cells and NK cells in vitro. The results showed that ADR T cells could selectively eliminate activated T cells and NK cells through 4-1BB in vitro, but had no effect on resting immune cells (Figure 1).
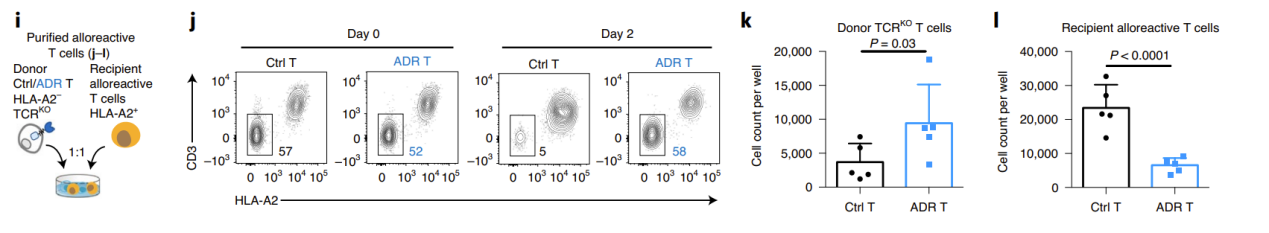
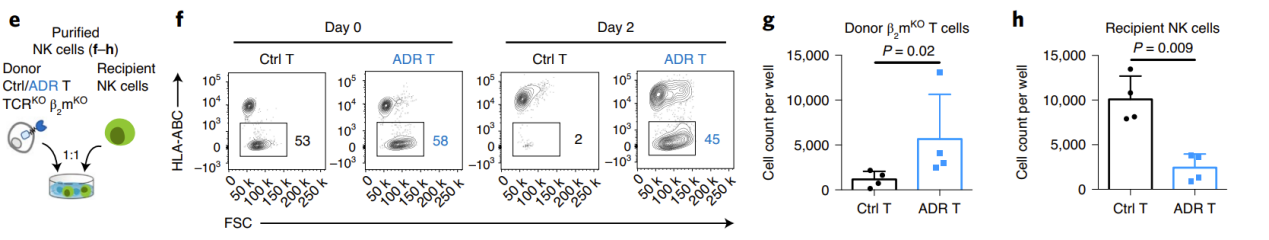
Next, the researchers used the mixed lymphocyte reaction (MLR) model to uate the ability of ADR T cells to eliminate allogeneic killer cells and resist immune rejection in vitro. To minimize GvHD, the researchers knocked out the donor TCR. In the NK-mediated immune rejection model, the researchers simultaneously blocked the β2m of the donor T cells, causing the loss of MHC-I molecules, thereby inhibiting T cell recognition and promoting NK cell activation. The results showed that on days 9-12 of co-incubation with allogeneic immune cells, the donor T cells in the control group were eliminated, while the ADR T cells significantly expanded. At the same time, the receptor lymphocytes in the ADR T cell treatment group were significantly controlled (Figure 2).
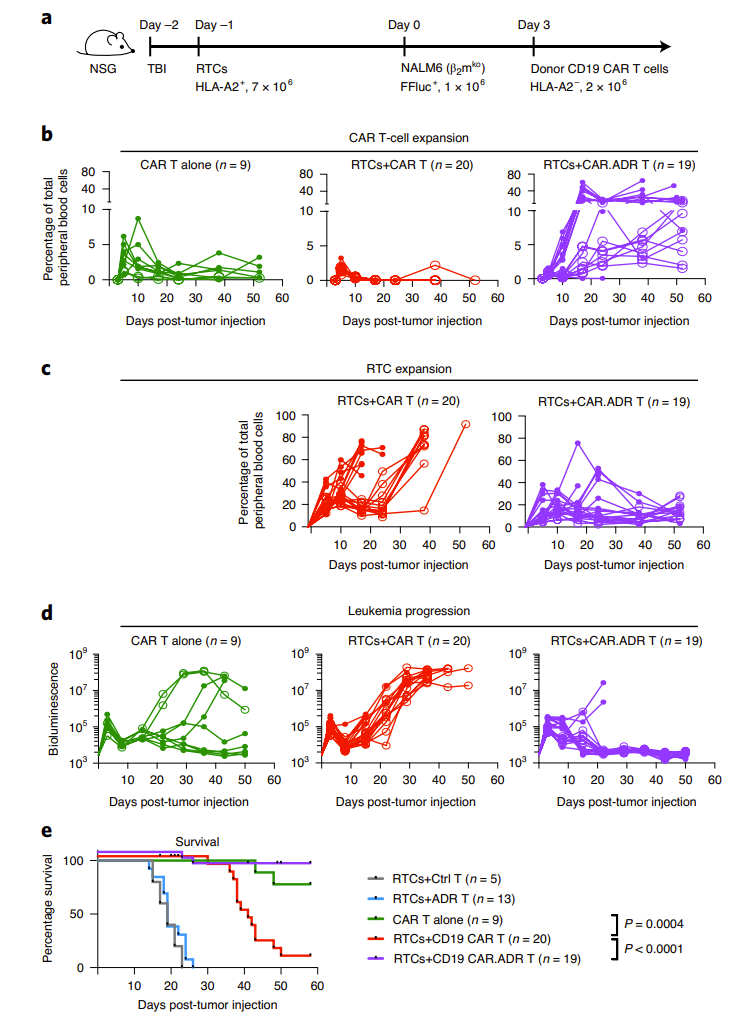
Subsequently, the researchers established a mouse model of allogeneic immune rejection. They first used a semi-lethal dose of rays to eliminate mouse lymphocytes, then reinfused recipient T cells or PBMC, and 4 days later, reinfused allogeneic donors. cell. At 18 days/14 days, the donor cells in the control group had been completely eliminated, while the ADR T cells expanded significantly and effectively resisted immune rejection by allogeneic T cells and NK cells (Figure 3).

In addition, researchers have demonstrated in vitro experiments that CD19 co-expressing ADR CAR After co-culture of T (CAR ADR T) cells with target cell NALM6 and activated allogeneic T cells, they all maintained their respective active functions (Figure 4).

The researchers also established a B-cell lymphoma model based on the animal immune rejection model. They found that in the absence of receptor T cell RTCs (i.e., immune rejection), CD19 CAR The T cells were able to eliminate pre-established tumors in most mice and for a long time. However, in the presence of RTCs, CD19 CAR T cells are rapidly rejected in 95% of animal models, leading to the expansion of RTCs in peripheral blood. CD19 CAR-T cell therapy only exerts anti-tumor effects in a short period of time, followed by rapid recurrence of cancer in almost all mice and eventual death. . However, in CAR ADR T cell therapy, the CAR in the peripheral blood of RTCs-treated mice T cells proliferated significantly, and RTCs were observed to exhibit significant inhibition. This treatment method fully controlled the progression of lymphoma, and the survival period of mice was significantly longer than that of other groups (Figure 5).
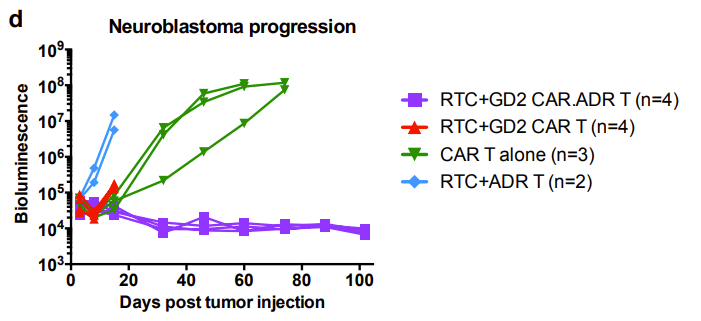
Similarly, the researchers demonstrated in a mouse xenograft model that GD2 CAR.ADR T cells were able to sustainably eradicate systemic neuroblastoma and resist rejection by the allogeneic immune system (Figure 6).
Finally, the researchers constructed an off-the-shelf T cell product that reduced GVHD by knocking out the donor cell TCR and combined ADR with CAR The results were consistent with the previous results, with lymphoma eradication in 20 out of 21 mice. In the control group without ADR, 22 out of 23 mice had rapid tumor recurrence and died (Figure 7).


In general, this study developed for the first time an ADR molecule that targets 4-1BB to address the problem of immune rejection mediated by T cells and NK cells during allogeneic cell therapy. This chimeric receptor can not only prolong the duration and anti-tumor function of therapeutic cells in non-immunocompromised patients, but also does not require ablation of lymphocyte components. This study provides new ideas for the development of off-the-shelf cell therapy products and provides a simple and effective way to overcome immune rejection.
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies and let more people know about the new development of biomedicine. The content of this article is only used for information exchange. This platform is neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information of this article shall not be used for diagnosis or treatment, can not substitute for professional medical opinion, the company website will not bear any responsibility. The final interpretation of the above statement belongs to the company website, this statement will apply to all the time shared articles, thank you for your cooperation! Copyright: The copyright of the article belongs to Shenzhen Cell Valley, and individuals are welcome to forward it to the circle of friends. Media or institutions, who will reprint it to other platforms in any form without authorization, will be regarded as infringement. If you need to reprint, please contact the email address: contact@sz-cell.com



