Research progress of universal cell therapy
CAR-T cell therapy brings new hope to cancer patients with its unique targeted therapy. The 10 CAR-T drugs currently on the market are mainly autologous CAR-T cell transplant treatments for CD19 or BCMA. According to statistics, only more than 35,000 patients have been treated in total, which is a drop in the bucket compared to the total number of patients in the world. . One of the most important reasons is that personalized customization makes the price unfriendly to the people. In addition, immune cell therapies are currently all autologous transplant treatments, so clinical treatments also face long preparation times. After bridging treatment, patients need to clear their lymph nodes and return to a physical state suitable for reinfusion of CAR-T. What's more important is that due to the effects of autoimmunity and chemotherapy, some patients' autologous T cells will experience exhaustion, aging, and functional defects. It is not enough to prepare sufficient CAR-T cells in vitro, thus affecting the final treatment effect. The cell therapy industry is rapidly implementing technology iterations and moving towards commercialization to cope with the huge market demand. In particular, there are technical barriers to the underlying technology viral vectors and efficient gene editing. They face pain points such as long R&D cycles, high production costs, and low conversion rates, which result in limited production capacity and high prices, making their commercialization path extremely challenging. Therefore, it is particularly important to develop universal cell products.
In order to allow more patients to benefit from immune cell therapy, researchers are now developing safe and effective allogeneic transplant immune cells and preparing universal CAR-immune cells. The successful development of such products will greatly reduce production costs, shorten production time and better ensure product efficacy, benefiting more patients. The most important challenges of immune cell allogeneic transplantation are graft-versus-host disease (GvHD) and host-versus-graft reaction (HvGR). To address this challenge, researchers mainly address this challenge from the following aspects [1]:
1.T cells can destroy GvHD by knocking out TRAC, and other additional gene editing can resist host rejection, such as modification of graft MHC-I related sites (B2M-HLA I); it can also be done through alemtuzumab The combined use of drugs inhibits the CD52 target and escapes the killing of allogeneic NK cells [2].

Figure 1. Universal CAR-T treatment strategy [8]
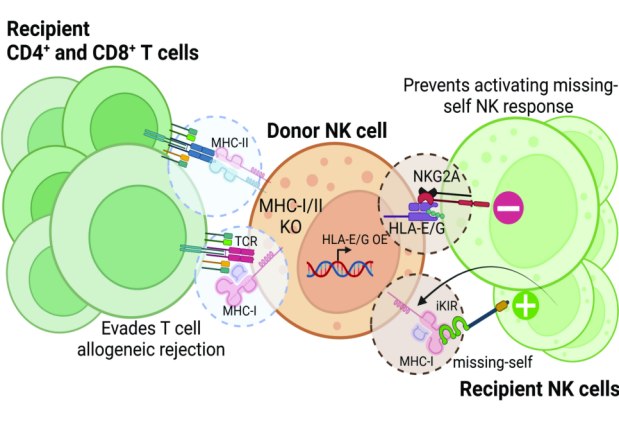
Figure 2. Universal NK cell strategy [7]

Shenzhen Cell Valley has achieved breakthroughs in the preparation process of NK, CAR-NK, γδT and CAR-γδT, especially in CAR-NK. Interested readers can review the scientific research enabling of 20231202, and welcome readers to communicate and guide our company to jointly promote the advent of new general-purpose cell products!
Reference:
[1] Ruella, Marco et al. Mechanisms of resistance to chimeric antigen receptor-T cells in haematological malignancies. Nature reviews. Drug discovery vol. 22,12 (2023): 976-995.
[2] Benjamin, R. et al. UCART19, a first-in-class allogeneic anti-CD19 chimeric antigen receptor T-cell therapy for adults with relapsed or refractory B-cell acute lymphoblastic leukaemia (CALM): a phase 1, dose-escalation trial. Lancet Haematol. 9, e833–e843 (2022)
[3] Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
[4] Mo, F. et al. Engineered of-the-shelf therapeutic T cells resist host immune rejection. Nat. Biotechnol. 39, 56–63 (2021).
[5] Nitsche, A. et al. Cytokine profiles of cord and adult blood leukocytes: diferences in expression are due to diferences in expression and activation of transcription factors. BMC Immunol. 8, 18 (2007)
[6] Merino, Aimee et al. Advances in NK cell therapy for hematologic malignancies: NK source, persistence and tumor targeting. Blood reviews vol. 60, 101073 (2023).
[7] Berrien-Elliott, Melissa M et al. Allogeneic natural killer cell therapy. Blood vol. 141,8 (2023): 856-868.
[8] Sadeqi Nezhad, et al. Induced Pluripotent Stem Cells (iPSCs) Provide a Potentially Unlimited T Cell Source for CAR-T Cell Development and Off-the-Shelf Products. Pharmaceutical Research. 38 (2021).
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies, so that more people understand the new development of biomedicine. The content of this article is only used for information exchange, and the platform remains neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information in this article should not be used as a diagnosis or treatment, is not a substitute for professional medical advice, and the company's website will not assume any responsibility. The final interpretation of the content of the above statement belongs to the company's website, this statement will apply to the company's website all the time to share the article, thank you for your cooperation! Copyright description: The copyright of the article belongs to Shenzhen Cell Valley, individuals are welcome to forward to the circle of friends, media or institutions without authorization, reproduced in any form to other platforms, will be regarded as infringement. For reprinting, please contact email: contact@sz-cell.com
In order to allow more patients to benefit from immune cell therapy, researchers are now developing safe and effective allogeneic transplant immune cells and preparing universal CAR-immune cells. The successful development of such products will greatly reduce production costs, shorten production time and better ensure product efficacy, benefiting more patients. The most important challenges of immune cell allogeneic transplantation are graft-versus-host disease (GvHD) and host-versus-graft reaction (HvGR). To address this challenge, researchers mainly address this challenge from the following aspects [1]:
1.T cells can destroy GvHD by knocking out TRAC, and other additional gene editing can resist host rejection, such as modification of graft MHC-I related sites (B2M-HLA I); it can also be done through alemtuzumab The combined use of drugs inhibits the CD52 target and escapes the killing of allogeneic NK cells [2].
2. Selecting γδ T cells, NK cells, etc. from healthy donors can avoid or reduce GvHD.
Based on the above theory, we summarize the research progress of researchers on universal cell therapy:
一、Universal CAR-T and TCR-T cells
Researchers used CRISPR-Cas9 to integrate CD19-CAR into the TRAC site in order to knock out the TCR of the donor cells and introduce specific CAR molecules targeting CD19 at the same time, so that the transplantation of universal CAR-T cells can avoid GVHD. At the same time, CD19-CAR is expressed under the natural TCR promoter. This genetic engineering modification strengthens the function of CAR-T cells and better controls the disease progression of pre-B-ALL [3]. However, there are also related problems. For example, among the 21 patients treated with 'UCART19', 14 patients achieved complete remission or complete remission with incomplete blood recovery 28 days after infusion, but 4 of them did not receive No significant UCART19 amplification occurred in patients treated with CD52-targeting alemtuzumab [2]. Therefore, host rejection of allogeneic CAR-T remains a major factor. The main way to prevent allogeneic cells from being rejected by the host is to prevent autologous immune cells from recognizing the transplanted cells as foreign cells and killing them. For example, Caribou bioscience's CB011 and CB012 series both knock out B2M in donor cells and insert B2M-HLA-E to escape the attack of host T lymphocytes and NK cells. Some researchers have also constructed an alloimmune defense receptor (ADR) through genetic engineering, which can selectively recognize 4-1BB. 4-1BB is a cell surface receptor that is transiently upregulated on the surface of activated lymphocytes and expresses ADR. CAR-T cells can effectively resist alloreactive T cells both in vivo and in vitro. In hematological tumors and solid tumor mouse models, ADR-expressing allogeneic CD19-CAR-T treatment has shown durable tumor elimination [4] . This method may have good prospects for the development of universal CAR-T in the future.
Based on the above theory, we summarize the research progress of researchers on universal cell therapy:
一、Universal CAR-T and TCR-T cells
Researchers used CRISPR-Cas9 to integrate CD19-CAR into the TRAC site in order to knock out the TCR of the donor cells and introduce specific CAR molecules targeting CD19 at the same time, so that the transplantation of universal CAR-T cells can avoid GVHD. At the same time, CD19-CAR is expressed under the natural TCR promoter. This genetic engineering modification strengthens the function of CAR-T cells and better controls the disease progression of pre-B-ALL [3]. However, there are also related problems. For example, among the 21 patients treated with 'UCART19', 14 patients achieved complete remission or complete remission with incomplete blood recovery 28 days after infusion, but 4 of them did not receive No significant UCART19 amplification occurred in patients treated with CD52-targeting alemtuzumab [2]. Therefore, host rejection of allogeneic CAR-T remains a major factor. The main way to prevent allogeneic cells from being rejected by the host is to prevent autologous immune cells from recognizing the transplanted cells as foreign cells and killing them. For example, Caribou bioscience's CB011 and CB012 series both knock out B2M in donor cells and insert B2M-HLA-E to escape the attack of host T lymphocytes and NK cells. Some researchers have also constructed an alloimmune defense receptor (ADR) through genetic engineering, which can selectively recognize 4-1BB. 4-1BB is a cell surface receptor that is transiently upregulated on the surface of activated lymphocytes and expresses ADR. CAR-T cells can effectively resist alloreactive T cells both in vivo and in vitro. In hematological tumors and solid tumor mouse models, ADR-expressing allogeneic CD19-CAR-T treatment has shown durable tumor elimination [4] . This method may have good prospects for the development of universal CAR-T in the future.

Figure 1. Universal CAR-T treatment strategy [8]
二、Universal NK and CAR-NK cells
Studies have found that, compared with peripheral blood stem cell transplantation, patients receiving allogeneic cord blood transplantation have lower levels of pro-inflammatory cytokines, significantly reducing GVHD[5]. Other sources of immune effector cells, such as induced pluripotent stem cells, are also being explored.
NK cells are a type of cytotoxic cells that play an important role in the innate immune response against viral or bacterial infections or damaged cells. Since they do not exert their killing function by recognizing allogeneic HLA and will not cause GVHD, NK cells have the potential to be used as allogeneic cell immunotherapy. NK cell activation is regulated by a variety of transmembrane receptors, including activating receptors, inhibitory receptors, cytokine receptors and chemokine receptors. MHC downregulation is a common feature of tumor cells, which provides an activation and killing mechanism for NK cells. When the inhibitory killer immunoglobulin receptor KIR on the surface of NK cells binds to tumor cells with downregulated MHC-I, the NK inhibitory signal is weakened, thereby inducing the killing function of NK cells. The inhibitory receptor CD94 (NKG2A or NKG2C heterodimer) on the surface of NK cells can recognize non-classical HLA-E molecules. Malignant tumors or virus-infected cells can escape the killing of the immune system through this molecular pathway. The activation signal of NKG2C can activate NK cells to perform killing functions, so NKG2C-positive NK cells can better enable NK cells to resist the escape of HLA-E-positive tumors. In addition, in addition to the natural killing ability of NK cells, CAR-NK can also specifically recognize tumor antigens through CAR molecules and exert anti-tumor effects [6]. However, allogeneic NK cells are still susceptible to allogeneic rejection by the host immune system. The most commonly used solution is to genetically knock out HLA-I class molecules (B2M genes) in NK cells and express single-chain HLA-E molecules to escape the killing of host T cells, NK and macrophages, and also to prevent transplantation NK cells kill each other [7].
Studies have found that, compared with peripheral blood stem cell transplantation, patients receiving allogeneic cord blood transplantation have lower levels of pro-inflammatory cytokines, significantly reducing GVHD[5]. Other sources of immune effector cells, such as induced pluripotent stem cells, are also being explored.
NK cells are a type of cytotoxic cells that play an important role in the innate immune response against viral or bacterial infections or damaged cells. Since they do not exert their killing function by recognizing allogeneic HLA and will not cause GVHD, NK cells have the potential to be used as allogeneic cell immunotherapy. NK cell activation is regulated by a variety of transmembrane receptors, including activating receptors, inhibitory receptors, cytokine receptors and chemokine receptors. MHC downregulation is a common feature of tumor cells, which provides an activation and killing mechanism for NK cells. When the inhibitory killer immunoglobulin receptor KIR on the surface of NK cells binds to tumor cells with downregulated MHC-I, the NK inhibitory signal is weakened, thereby inducing the killing function of NK cells. The inhibitory receptor CD94 (NKG2A or NKG2C heterodimer) on the surface of NK cells can recognize non-classical HLA-E molecules. Malignant tumors or virus-infected cells can escape the killing of the immune system through this molecular pathway. The activation signal of NKG2C can activate NK cells to perform killing functions, so NKG2C-positive NK cells can better enable NK cells to resist the escape of HLA-E-positive tumors. In addition, in addition to the natural killing ability of NK cells, CAR-NK can also specifically recognize tumor antigens through CAR molecules and exert anti-tumor effects [6]. However, allogeneic NK cells are still susceptible to allogeneic rejection by the host immune system. The most commonly used solution is to genetically knock out HLA-I class molecules (B2M genes) in NK cells and express single-chain HLA-E molecules to escape the killing of host T cells, NK and macrophages, and also to prevent transplantation NK cells kill each other [7].

Figure 2. Universal NK cell strategy [7]
三、General type γδT and CAR-γδT cells
T cells can be divided into αβT cells and γδT cells according to the difference of TCR. Human peripheral blood lymphocytes are mainly αβT cells, and γδT cells generally account for only 1%-5%. Although the proportion of γδT cells is small, γδT cells can directly kill tumor cells through the NK cell receptor on the cell surface, ADCC effect and secreted cytokines (IFN-γ, TNF-α). Moreover, γδT cells can also act as antigen-presenting cells to activate αβT cells, or induce anti-tumor cytotoxicity of NK cells by 4-1BB co-stimulation pathway, so as to achieve indirect killing of tumors. Moreover, γδT cells do not present the risk of GvHD during allogeneic cell transplantation, so they are safer. In addition to the natural anti-tumor effect, CAR-γδT can further specifically recognize tumor-associated antigens through CAR molecules, and play a direct role in killing tumors.
T cells can be divided into αβT cells and γδT cells according to the difference of TCR. Human peripheral blood lymphocytes are mainly αβT cells, and γδT cells generally account for only 1%-5%. Although the proportion of γδT cells is small, γδT cells can directly kill tumor cells through the NK cell receptor on the cell surface, ADCC effect and secreted cytokines (IFN-γ, TNF-α). Moreover, γδT cells can also act as antigen-presenting cells to activate αβT cells, or induce anti-tumor cytotoxicity of NK cells by 4-1BB co-stimulation pathway, so as to achieve indirect killing of tumors. Moreover, γδT cells do not present the risk of GvHD during allogeneic cell transplantation, so they are safer. In addition to the natural anti-tumor effect, CAR-γδT can further specifically recognize tumor-associated antigens through CAR molecules, and play a direct role in killing tumors.

Figure 3. Anti-tumor mechanism of γδT cells [9]
Shenzhen Cell Valley has achieved breakthroughs in the preparation process of NK, CAR-NK, γδT and CAR-γδT, especially in CAR-NK. Interested readers can review the scientific research enabling of 20231202, and welcome readers to communicate and guide our company to jointly promote the advent of new general-purpose cell products!
Reference:
[1] Ruella, Marco et al. Mechanisms of resistance to chimeric antigen receptor-T cells in haematological malignancies. Nature reviews. Drug discovery vol. 22,12 (2023): 976-995.
[2] Benjamin, R. et al. UCART19, a first-in-class allogeneic anti-CD19 chimeric antigen receptor T-cell therapy for adults with relapsed or refractory B-cell acute lymphoblastic leukaemia (CALM): a phase 1, dose-escalation trial. Lancet Haematol. 9, e833–e843 (2022)
[3] Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
[4] Mo, F. et al. Engineered of-the-shelf therapeutic T cells resist host immune rejection. Nat. Biotechnol. 39, 56–63 (2021).
[5] Nitsche, A. et al. Cytokine profiles of cord and adult blood leukocytes: diferences in expression are due to diferences in expression and activation of transcription factors. BMC Immunol. 8, 18 (2007)
[6] Merino, Aimee et al. Advances in NK cell therapy for hematologic malignancies: NK source, persistence and tumor targeting. Blood reviews vol. 60, 101073 (2023).
[7] Berrien-Elliott, Melissa M et al. Allogeneic natural killer cell therapy. Blood vol. 141,8 (2023): 856-868.
[8] Sadeqi Nezhad, et al. Induced Pluripotent Stem Cells (iPSCs) Provide a Potentially Unlimited T Cell Source for CAR-T Cell Development and Off-the-Shelf Products. Pharmaceutical Research. 38 (2021).
[9]Yuan Song, et al. Targeting Cytokine Signals to Enhance γδT Cell-Based Cancer Immunotherapy. Front. Immunol., (2022)
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies, so that more people understand the new development of biomedicine. The content of this article is only used for information exchange, and the platform remains neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information in this article should not be used as a diagnosis or treatment, is not a substitute for professional medical advice, and the company's website will not assume any responsibility. The final interpretation of the content of the above statement belongs to the company's website, this statement will apply to the company's website all the time to share the article, thank you for your cooperation! Copyright description: The copyright of the article belongs to Shenzhen Cell Valley, individuals are welcome to forward to the circle of friends, media or institutions without authorization, reproduced in any form to other platforms, will be regarded as infringement. For reprinting, please contact email: contact@sz-cell.com


