Study on CD123-targeted CAR-T cells for the treatment of AML
CAR-T cell therapy is an immunotherapy method that uses genetic engineering technology to modify human T cells so that they can specifically recognize and kill tumor cells. It is a tumor immunotherapy method that has received widespread attention in recent years, especially in hematological tumors. It has shown good results. The nine CAR-T products currently on the market are mainly used to treat B-cell leukemia, B-cell lymphoma and multiple myeloma. CAR-T products for acute myeloid leukemia (AML) still need to be developed urgently.
AML is the most common type of acute leukemia in adults and the leading cause of death from leukemia each year. Although complete remission can be achieved with intensive consolidation chemotherapy regimens or stem cell transplantation, the vast majority of patients will relapse. Relapse can be attributed to a relatively rare, chemotherapy-resistant cell subset called leukemic stem cells (LSCs), which can self-renew, proliferate, and differentiate into leukemic blasts. Studies have shown that patients with a higher proportion of phenotypically defined LSC (CD34+CD38-) have a significantly lower recurrence-free survival rate than patients with a low proportion of LSC. While many chemotherapy drugs can kill leukemia blasts, no chemotherapy drug routinely used in AML treatment can eliminate LSCs. In summary, LSCs play a key role in disease recurrence, cannot be ablated by existing treatments, and are highly relevant therapeutic targets for AML.
In a new preclinical study, Weill Cornell Medical College researchers found that their drug UCART123 can successfully target specific cancer cells that may cause AML to relapse, known as leukemia stem cells, confirming the approach. Effectiveness in animal models of acute myeloid leukemia. This new cell therapy, currently in Phase 1 clinical trials, may help AML patients remain cancer-free. The relevant research results were published in the journal "Nature Communications" with the title of "Allogeneic TCRαβ deficient CAR T-cells targeting CD123 in acute myeloid leukemia".

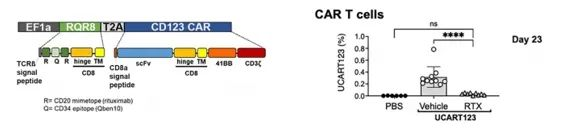
In this study, the authors used T cells from third-party healthy donors to generate allogeneic chimeric antigen receptor T cells to generate universal CAR T cells targeting CD123 (UCART123). UCART123 does not express TcRαβ, and has been pre-treated with knockout of the TcRα constant gene (Trac) and elimination of remaining TcRαβ-positive cells to minimize the possibility of graft-versus-host disease (GvHD). In addition, the CAR construct was co-expressed with RQR8 as a safety switch to allow elimination of CAR-T cells in the presence of rituximab.
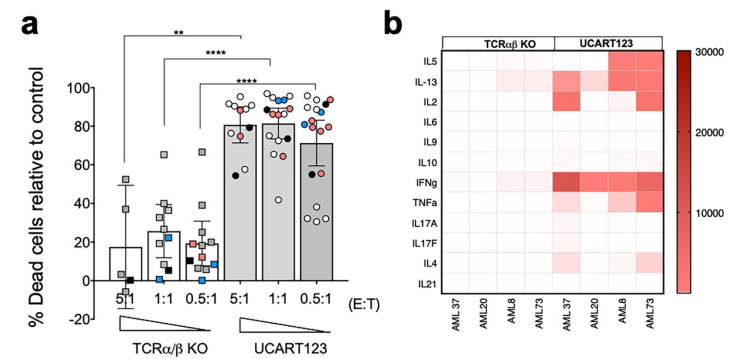
The authors confirmed the specific antitumor activity of UCART123 against CD123+ primary AML samples by in vitro cytotoxicity assays and colony formation assays. UCART123 induced specific lysis of all AML samples tested. In addition, we found that several cytokines, such as IFN-γ, IL-2, IL-13, TNF-α, and IL-5, were produced when UCART123 was cultured with all acute myeloid leukemia samples. Importantly, primary leukemia samples were unable to form colony units after UCART123 treatment, indicating their ability to eliminate leukemic progenitor cells.

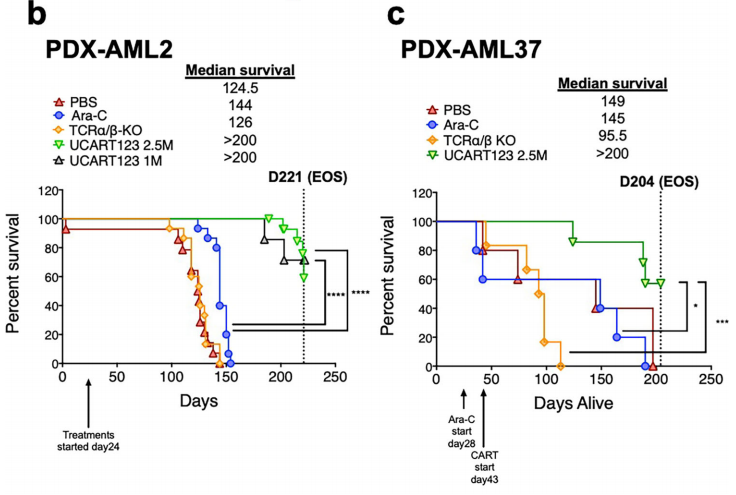
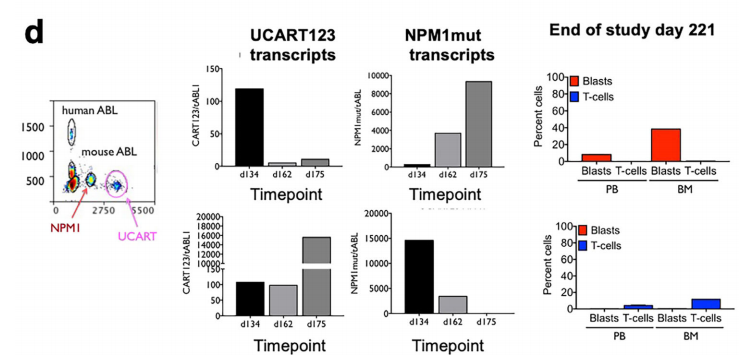
The authors also uated the selective killing activity of UCART123 in vivo. In PDX-AML mice, human CD34+ cells from cord blood or lymphocyte-depleted bone marrow were used for detection and found that UCART123-treated mice carrying human AML (PDX-AML) The overall survival rate of mice was significantly improved. To monitor residual disease and persistence of UCART cells, we developed a ddPCR method to simultaneously uate these two cell populations and used NPM1-mutated AML as a model to verify the above conclusion. The assay demonstrated that continued detection of UCART123 maintained the health of the mice, even when AML cells challenged them again.
Finally, the authors established a competitive NBM/AML model in which leukemia cells compete with normal hematopoietic cells to simulate what occurs in leukemia patients. found that in a competitive NBM/AML model, UCART123 preferentially targeted AML cells and significantly eliminated AML cells on day 16 after treatment.
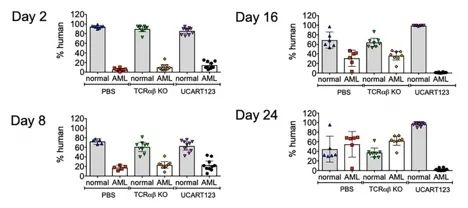
In summary, the authors used primary AML samples, normal bone marrow (BM) and cord blood (CB) cells, patient-derived xenografts (PDX), normal bone marrow (NBM) humanized xenografts (HU-X) models and competitive NBM/AML xenograft model to test the activity of UCART123 in vitro and in vivo. Data show that UCART123 can eliminate LSCs and preferentially eliminate AML cells in vitro and in vivo without significant effects on normal cells, which is expected to prevent the recurrence of AML leukemia.
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies and let more people know about the new development of biomedicine. The content of this article is only used for information exchange. This platform is neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information of this article shall not be used for diagnosis or treatment, can not substitute for professional medical opinion, the company website will not bear any responsibility. The final interpretation of the above statement belongs to the company website, this statement will apply to all the time shared articles, thank you for your cooperation! Copyright: The copyright of the article belongs to Shenzhen Cell Valley, and individuals are welcome to forward it to the circle of friends. Media or institutions, who will reprint it to other platforms in any form without authorization, will be regarded as infringement. If you need to reprint, please contact the email address: contact@sz-cell.com
AML is the most common type of acute leukemia in adults and the leading cause of death from leukemia each year. Although complete remission can be achieved with intensive consolidation chemotherapy regimens or stem cell transplantation, the vast majority of patients will relapse. Relapse can be attributed to a relatively rare, chemotherapy-resistant cell subset called leukemic stem cells (LSCs), which can self-renew, proliferate, and differentiate into leukemic blasts. Studies have shown that patients with a higher proportion of phenotypically defined LSC (CD34+CD38-) have a significantly lower recurrence-free survival rate than patients with a low proportion of LSC. While many chemotherapy drugs can kill leukemia blasts, no chemotherapy drug routinely used in AML treatment can eliminate LSCs. In summary, LSCs play a key role in disease recurrence, cannot be ablated by existing treatments, and are highly relevant therapeutic targets for AML.
In a new preclinical study, Weill Cornell Medical College researchers found that their drug UCART123 can successfully target specific cancer cells that may cause AML to relapse, known as leukemia stem cells, confirming the approach. Effectiveness in animal models of acute myeloid leukemia. This new cell therapy, currently in Phase 1 clinical trials, may help AML patients remain cancer-free. The relevant research results were published in the journal "Nature Communications" with the title of "Allogeneic TCRαβ deficient CAR T-cells targeting CD123 in acute myeloid leukemia".

In this study, the authors used T cells from third-party healthy donors to generate allogeneic chimeric antigen receptor T cells to generate universal CAR T cells targeting CD123 (UCART123). UCART123 does not express TcRαβ, and has been pre-treated with knockout of the TcRα constant gene (Trac) and elimination of remaining TcRαβ-positive cells to minimize the possibility of graft-versus-host disease (GvHD). In addition, the CAR construct was co-expressed with RQR8 as a safety switch to allow elimination of CAR-T cells in the presence of rituximab.

The authors confirmed the specific antitumor activity of UCART123 against CD123+ primary AML samples by in vitro cytotoxicity assays and colony formation assays. UCART123 induced specific lysis of all AML samples tested. In addition, we found that several cytokines, such as IFN-γ, IL-2, IL-13, TNF-α, and IL-5, were produced when UCART123 was cultured with all acute myeloid leukemia samples. Importantly, primary leukemia samples were unable to form colony units after UCART123 treatment, indicating their ability to eliminate leukemic progenitor cells.


The authors also uated the selective killing activity of UCART123 in vivo. In PDX-AML mice, human CD34+ cells from cord blood or lymphocyte-depleted bone marrow were used for detection and found that UCART123-treated mice carrying human AML (PDX-AML) The overall survival rate of mice was significantly improved. To monitor residual disease and persistence of UCART cells, we developed a ddPCR method to simultaneously uate these two cell populations and used NPM1-mutated AML as a model to verify the above conclusion. The assay demonstrated that continued detection of UCART123 maintained the health of the mice, even when AML cells challenged them again.


Finally, the authors established a competitive NBM/AML model in which leukemia cells compete with normal hematopoietic cells to simulate what occurs in leukemia patients. found that in a competitive NBM/AML model, UCART123 preferentially targeted AML cells and significantly eliminated AML cells on day 16 after treatment.

In summary, the authors used primary AML samples, normal bone marrow (BM) and cord blood (CB) cells, patient-derived xenografts (PDX), normal bone marrow (NBM) humanized xenografts (HU-X) models and competitive NBM/AML xenograft model to test the activity of UCART123 in vitro and in vivo. Data show that UCART123 can eliminate LSCs and preferentially eliminate AML cells in vitro and in vivo without significant effects on normal cells, which is expected to prevent the recurrence of AML leukemia.
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies and let more people know about the new development of biomedicine. The content of this article is only used for information exchange. This platform is neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information of this article shall not be used for diagnosis or treatment, can not substitute for professional medical opinion, the company website will not bear any responsibility. The final interpretation of the above statement belongs to the company website, this statement will apply to all the time shared articles, thank you for your cooperation! Copyright: The copyright of the article belongs to Shenzhen Cell Valley, and individuals are welcome to forward it to the circle of friends. Media or institutions, who will reprint it to other platforms in any form without authorization, will be regarded as infringement. If you need to reprint, please contact the email address: contact@sz-cell.com



