Limitations and breakthrough directions of CAR-T in the treatment of solid tumors
The incidence and mortality of cancer continue to increase, but effective treatments have not yet been found. Traditional therapies, including surgery, radiotherapy and chemotherapy, have major limitations in cancer treatment and face problems such as metastasis, recurrence and poor efficacy.
In the past decade, based on the in-depth development of molecular medicine and immunology, various targeted therapies have made great progress in cancer. Among them, tumor immunotherapy has gradually become a research hotspot, providing potential therapeutic strategies for cancer treatment.
In particular , the remarkable efficacy of chimeric antigen receptor CAR-T cell therapy in hematomas is revolutionary. Currently, 9 CAR-T products are on the market for the treatment of hematomas worldwide. Compared with hematological tumors, solid tumors have the characteristics of strong tumor heterogeneity, limited target antigen selection, low T cell infiltration rate, and immunosuppressive tumor microenvironment (TME). Therefore, the application of CAR-T in solid tumors is not optimistic. The main reasons are:
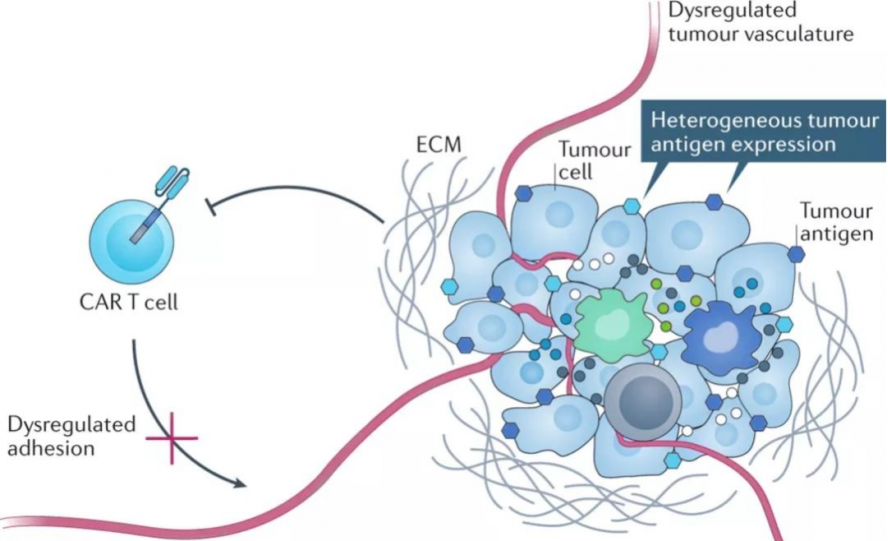
(1) Complex tumor microenvironment and T cell exhaustion
Research shows that the tumor microenvironment (TME) has low pH, low oxygen, high permeability, and an immunosuppressive mechanism, which is extremely unfavorable for T cell survival and immune efficacy. At the same time, there are many cells that drive immune suppression near solid tumors, including myeloid-derived suppressor cells (MDSC), tumor-associated macrophages (TAM), and regulatory T cells (Treg). They can infiltrate solid tumors and drive tumors to secrete cytokines such as growth factors and chemokines [1] . The interaction of tumor cells with the immune system promotes an immunosuppressive microenvironment that reduces anti-tumor immune activity through immune checkpoint pathways, such as PD-1 or CTLA-4.
CAR-T in solid tumors is hindered is T cell exhaustion. Exhausted T cells experience a progressive loss of effector function: in the early stages of T cell exhaustion, T cell activation factors, such as IL-2 and TNF-α, promote tumor cell killing function defects; in the exhaustion stage, IFN-γ production is lost, and T cells lose their proliferation ability and cytotoxicity [2] . In addition, exhausted T cells can continue to express inhibitory immune checkpoint receptors, such as PD-1, TIM-3, and CTLA-4, becoming "accomplices" of tumor immune escape. Exhausted T cells have reduced mitochondrial activity, limited glycolysis, and correspondingly reduced metabolic reserve capacity [3] .
This T cell failure is triggered by a combination of different inhibitory pathways. Therefore, combined immunotherapy with CAR-T cells and immune checkpoint blockade is considered to be at the forefront of immunotherapy development.
(2) Antigenic escape
One of the most challenging limitations of CAR-T cell therapy is the development of tumor resistance to single-antigen-targeting CAR constructs. In CAR-T treatment of hematological tumors, initial single-antigen-targeting CAR-T cells can provide high response rates, but in some patients who receive treatment, whether targeting CD19 [4] or BCMA [5] , performance Partial or complete loss of target antigen expression is a phenomenon known as antigen escape. Similarly, patterns of antigen escape resistance have also emerged in solid tumors. Solid tumors have significant antigenic heterogeneity. There may be significant differences between individual patients or between tumor cells in different parts of the same patient's body. As a result, the same CAR-T may only kill some solid tumor cells, leaving the remaining Tumor cells continue to proliferate and metastasize, and researchers have found that bivalent CAR seems to be able to reduce antigen escape [6][7] .
(3) Off-target effect
Off-target effects, the full name of which is On-target off-tumor effects, are one of the major challenges for CAR-T targeting solid tumor antigens. There are very few tumor-specific antigens (TSA) in solid tumors. Most of the antigens found to be highly expressed in tumors are tumor-associated antigens (TAA), which are usually expressed in normal tissues. This brings a high off-target risk to CAR-T therapy, making the safety of CAR-T therapy a key issue. Therefore, antigen selection is crucial in CAR design, not only to ensure therapeutic efficacy but also to limit off-target toxicity.
(4) Migration and tumor infiltration of CAR-T cells
Compared with hematological malignancies, CAR-T cell therapy for solid tumors is limited by the ability of CAR-T cells to migrate and infiltrate into solid tumors. On the one hand, unlike blood tumor cells, which are dispersed, solid tumors often form solid masses with abundant tumor-associated fibroblasts (CAFs) and blood vessels, forming a natural physical barrier that makes CAR-T The lesion is difficult to reach. On the other hand, under normal circumstances, the interaction between chemokines and their receptors will promote the migration of T cells into the tumor microenvironment, but solid tumors can inhibit the secretion of certain chemokines, and at the same time, related receptors on the surface of CAR-T cells The lack of CAR-T together leads to insufficient homing ability of CAR-T to tumor sites.
(5) CAR-T cell related toxicity
Although CAR-T cell therapy is already a revolutionary cancer treatment tool, it has high toxic and side effects, such as cytokine release syndrome (CRS), hemophagocytic syndrome (HLH)/macrophage activation syndrome (MAS). ) and/or immune effector cell-associated neurotoxic syndrome (ICANS), or even death, hinders the further development of CAR-T cell therapy. Even in clinical trials with the highest response rates so far, patients can experience serious life-threatening events.
CAR-T cells are infused back into the patient's body and are activated and expanded under the action of tumor cells and the immune system. The body will release a large amount of cytokines, leading to cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). Toxic and side effects. It is worth noting that these cytokines are not exclusively released by CAR-T , and there is evidence that CRS and ICANS are mediated to a greater extent by IL-1 and IL-6 released by macrophages. CAR-T- mediated tumors Factors released by apoptosis are also involved in the occurrence and development of cytokine storm. In several clinical studies of CAR-T treatment of solid tumors, varying degrees of CRS have been observed, and ICANS has been found in brain gliomas. Therefore, controlling cytokine release is also one of the directions for future improvement of CAR-T therapy. [8] .
In summary, although there are many challenges in the application of CAR-T in solid tumors, new strategies and solutions are constantly being proposed. The solid tumor market is far broader than hematological tumors. More and more CAR-T companies are entering this field,and the number of clinical trials is also increasing. It is also used in the treatment of some solid tumors (breast cancer, non-small cell lung cancer, etc.) Major breakthroughs have also been made in [10][11] , and CAR-T therapy is likely to become a more effective and safer treatment for solid tumors in the future.
Reference Documentation
[1] Andrew J. Hou, et al. Navigating CAR-T cells through thesolid-tumour microenvironment. Nature Reviews Drug Discovery. (2021)
[2] PRITYKIN Y, et al. A unified atlas of CD8 T cell dysfunctional states in cancer and infection [J]. Mol Cel. (2021)
[3] MCGOWAN E, et al. PD-1 disrupted CAR-T cells in the treatment of solid tumors: Promises and challenges [J]. Biomed Pharmacother. (2020)
[4] Majzner, et al. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. (2018).
[5] Cohen, A. D. et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Invest. 129, 2210–2221 (2019).
[6] Koichi Hirabayashi, et al. Dual-targeting CAR-T cells with optimalco-stimulation and metabolic fitness enhance antitumor activity and preventescape in solid tumors. Nature Cancer. (2021)
[7] Evgin L, et al. Oncolytic virus-mediated expansion of dual-specific CAR T cells improves efficacy against solid tumors in mice. Sci Transl Med. (2022)
[8] Urvi P, et al. CAR T cell therapy in solid tumors: A review of current clinical trials. eJHaem. (2022)
[9] World Health Organization (WHO) Cancer [https:// www. who. int/ news- room/ fact- sheets/ detail/ cancer
[10] Patel U, et al. CAR T cell therapy in solid tumors: A review of current clinical trials. EJHaem. (2022)
[11] Maalej KM, et al. CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances. Mol Cancer. (2023)
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies and let more people know about the new development of biomedicine. The content of this article is only used for information exchange. This platform is neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information of this article shall not be used for diagnosis or treatment, can not substitute for professional medical opinion, the company website will not bear any responsibility. The final interpretation of the above statement belongs to the company website, this statement will apply to all the time shared articles, thank you for your cooperation! Copyright: The copyright of the article belongs to Shenzhen Cell Valley, and individuals are welcome to forward it to the circle of friends. Media or institutions, who will reprint it to other platforms in any form without authorization, will be regarded as infringement. If you need to reprint, please contact the email address: contact@sz-cell.com
In the past decade, based on the in-depth development of molecular medicine and immunology, various targeted therapies have made great progress in cancer. Among them, tumor immunotherapy has gradually become a research hotspot, providing potential therapeutic strategies for cancer treatment.
In particular , the remarkable efficacy of chimeric antigen receptor CAR-T cell therapy in hematomas is revolutionary. Currently, 9 CAR-T products are on the market for the treatment of hematomas worldwide. Compared with hematological tumors, solid tumors have the characteristics of strong tumor heterogeneity, limited target antigen selection, low T cell infiltration rate, and immunosuppressive tumor microenvironment (TME). Therefore, the application of CAR-T in solid tumors is not optimistic. The main reasons are:

(1) Complex tumor microenvironment and T cell exhaustion
Research shows that the tumor microenvironment (TME) has low pH, low oxygen, high permeability, and an immunosuppressive mechanism, which is extremely unfavorable for T cell survival and immune efficacy. At the same time, there are many cells that drive immune suppression near solid tumors, including myeloid-derived suppressor cells (MDSC), tumor-associated macrophages (TAM), and regulatory T cells (Treg). They can infiltrate solid tumors and drive tumors to secrete cytokines such as growth factors and chemokines [1] . The interaction of tumor cells with the immune system promotes an immunosuppressive microenvironment that reduces anti-tumor immune activity through immune checkpoint pathways, such as PD-1 or CTLA-4.
CAR-T in solid tumors is hindered is T cell exhaustion. Exhausted T cells experience a progressive loss of effector function: in the early stages of T cell exhaustion, T cell activation factors, such as IL-2 and TNF-α, promote tumor cell killing function defects; in the exhaustion stage, IFN-γ production is lost, and T cells lose their proliferation ability and cytotoxicity [2] . In addition, exhausted T cells can continue to express inhibitory immune checkpoint receptors, such as PD-1, TIM-3, and CTLA-4, becoming "accomplices" of tumor immune escape. Exhausted T cells have reduced mitochondrial activity, limited glycolysis, and correspondingly reduced metabolic reserve capacity [3] .
This T cell failure is triggered by a combination of different inhibitory pathways. Therefore, combined immunotherapy with CAR-T cells and immune checkpoint blockade is considered to be at the forefront of immunotherapy development.
(2) Antigenic escape
One of the most challenging limitations of CAR-T cell therapy is the development of tumor resistance to single-antigen-targeting CAR constructs. In CAR-T treatment of hematological tumors, initial single-antigen-targeting CAR-T cells can provide high response rates, but in some patients who receive treatment, whether targeting CD19 [4] or BCMA [5] , performance Partial or complete loss of target antigen expression is a phenomenon known as antigen escape. Similarly, patterns of antigen escape resistance have also emerged in solid tumors. Solid tumors have significant antigenic heterogeneity. There may be significant differences between individual patients or between tumor cells in different parts of the same patient's body. As a result, the same CAR-T may only kill some solid tumor cells, leaving the remaining Tumor cells continue to proliferate and metastasize, and researchers have found that bivalent CAR seems to be able to reduce antigen escape [6][7] .
(3) Off-target effect
Off-target effects, the full name of which is On-target off-tumor effects, are one of the major challenges for CAR-T targeting solid tumor antigens. There are very few tumor-specific antigens (TSA) in solid tumors. Most of the antigens found to be highly expressed in tumors are tumor-associated antigens (TAA), which are usually expressed in normal tissues. This brings a high off-target risk to CAR-T therapy, making the safety of CAR-T therapy a key issue. Therefore, antigen selection is crucial in CAR design, not only to ensure therapeutic efficacy but also to limit off-target toxicity.
(4) Migration and tumor infiltration of CAR-T cells
Compared with hematological malignancies, CAR-T cell therapy for solid tumors is limited by the ability of CAR-T cells to migrate and infiltrate into solid tumors. On the one hand, unlike blood tumor cells, which are dispersed, solid tumors often form solid masses with abundant tumor-associated fibroblasts (CAFs) and blood vessels, forming a natural physical barrier that makes CAR-T The lesion is difficult to reach. On the other hand, under normal circumstances, the interaction between chemokines and their receptors will promote the migration of T cells into the tumor microenvironment, but solid tumors can inhibit the secretion of certain chemokines, and at the same time, related receptors on the surface of CAR-T cells The lack of CAR-T together leads to insufficient homing ability of CAR-T to tumor sites.
(5) CAR-T cell related toxicity
Although CAR-T cell therapy is already a revolutionary cancer treatment tool, it has high toxic and side effects, such as cytokine release syndrome (CRS), hemophagocytic syndrome (HLH)/macrophage activation syndrome (MAS). ) and/or immune effector cell-associated neurotoxic syndrome (ICANS), or even death, hinders the further development of CAR-T cell therapy. Even in clinical trials with the highest response rates so far, patients can experience serious life-threatening events.
CAR-T cells are infused back into the patient's body and are activated and expanded under the action of tumor cells and the immune system. The body will release a large amount of cytokines, leading to cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). Toxic and side effects. It is worth noting that these cytokines are not exclusively released by CAR-T , and there is evidence that CRS and ICANS are mediated to a greater extent by IL-1 and IL-6 released by macrophages. CAR-T- mediated tumors Factors released by apoptosis are also involved in the occurrence and development of cytokine storm. In several clinical studies of CAR-T treatment of solid tumors, varying degrees of CRS have been observed, and ICANS has been found in brain gliomas. Therefore, controlling cytokine release is also one of the directions for future improvement of CAR-T therapy. [8] .
(6)expensive
In fact, according to data released by the WHO and cancer centers, the incidence of new cancer patients each year is less than 10% for hematologic malignancies, while the incidence of new solid tumors is as high as 90% [9] . However, while blockbuster drugs are constantly being released for hematologic malignancies, solid tumors are relatively unknown. In addition to the above limiting factors, price is also a challenge facing CAR-T therapy. Taking the currently available CAR-T drugs as an example, without considering the possibility of recurrence and ineffectiveness, a single injection of the drug costs more than 1 million yuan, not to mention the complexity of solid tumors compared to hematologic malignancies. Especially in China, CAR-T therapy is not yet included in medical insurance, which means that few families can afford this medical burden. The use of reverse transcription vector technology to prepare CAR-T can significantly reduce costs and drug prices, which may give more patients hope and stimulate various industries to accelerate the research and development of tumor immunotherapy.In summary, although there are many challenges in the application of CAR-T in solid tumors, new strategies and solutions are constantly being proposed. The solid tumor market is far broader than hematological tumors. More and more CAR-T companies are entering this field,and the number of clinical trials is also increasing. It is also used in the treatment of some solid tumors (breast cancer, non-small cell lung cancer, etc.) Major breakthroughs have also been made in [10][11] , and CAR-T therapy is likely to become a more effective and safer treatment for solid tumors in the future.
Reference Documentation
[1] Andrew J. Hou, et al. Navigating CAR-T cells through thesolid-tumour microenvironment. Nature Reviews Drug Discovery. (2021)
[2] PRITYKIN Y, et al. A unified atlas of CD8 T cell dysfunctional states in cancer and infection [J]. Mol Cel. (2021)
[3] MCGOWAN E, et al. PD-1 disrupted CAR-T cells in the treatment of solid tumors: Promises and challenges [J]. Biomed Pharmacother. (2020)
[4] Majzner, et al. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. (2018).
[5] Cohen, A. D. et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Invest. 129, 2210–2221 (2019).
[6] Koichi Hirabayashi, et al. Dual-targeting CAR-T cells with optimalco-stimulation and metabolic fitness enhance antitumor activity and preventescape in solid tumors. Nature Cancer. (2021)
[7] Evgin L, et al. Oncolytic virus-mediated expansion of dual-specific CAR T cells improves efficacy against solid tumors in mice. Sci Transl Med. (2022)
[8] Urvi P, et al. CAR T cell therapy in solid tumors: A review of current clinical trials. eJHaem. (2022)
[9] World Health Organization (WHO) Cancer [https:// www. who. int/ news- room/ fact- sheets/ detail/ cancer
[10] Patel U, et al. CAR T cell therapy in solid tumors: A review of current clinical trials. EJHaem. (2022)
[11] Maalej KM, et al. CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances. Mol Cancer. (2023)
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies and let more people know about the new development of biomedicine. The content of this article is only used for information exchange. This platform is neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information of this article shall not be used for diagnosis or treatment, can not substitute for professional medical opinion, the company website will not bear any responsibility. The final interpretation of the above statement belongs to the company website, this statement will apply to all the time shared articles, thank you for your cooperation! Copyright: The copyright of the article belongs to Shenzhen Cell Valley, and individuals are welcome to forward it to the circle of friends. Media or institutions, who will reprint it to other platforms in any form without authorization, will be regarded as infringement. If you need to reprint, please contact the email address: contact@sz-cell.com



