CAR T targeting intracellular oncoproteins
CAR - T cell tumor immunotherapy has demonstrated strong anti-tumor efficacy in hematological tumors such as leukemia, but its application in the field of solid tumors is limited due to the lack of tumor-specific targets. Most oncogenic driver genes are intracellular proteins or nuclear proteins that the immune system can recognize only through antigen peptides presented by the major histocompatibility complex (MHC), which limits the ability to target individual human leukocyte antigens (HLA). Immunotherapy with heterotypicly presented mutant peptides (neoantigens). The latest new study "Targeting of intracellular oncoproteins with peptide-centric CARs" published in the journal "Nature" directly allows CAR T cells to target tumor cells through peptide fragments of unmutated and essential proteins presented by MHC I, in part. Relevant research content is described below.

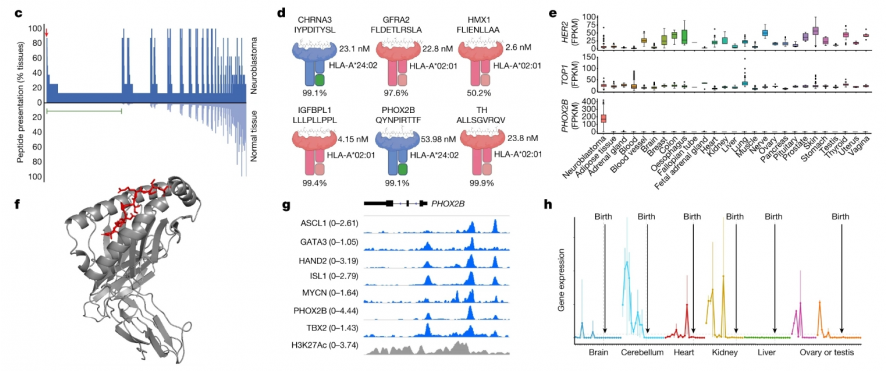
Researchers including John M. Maris of the University of Pennsylvania School of Medicine and Mark Yarmarkovich of the New York University School of Medicine first used mass spectrometry to analyze neuroblastoma (eight types of neuroblastoma) by performing MHC capture, peptide elution, and liquid chromatography and tandem mass spectrometry. (tumor cell-derived xenografts and patient-derived xenografts), and then compared the immune peptide group presented by MHC I and the immune peptide group potentially presented by normal tissue cells to screen for peptides specifically presented by the tumor, and finally determined the differences. Antigenic peptide PHOX2B. PHOX2B is only expressed in tumor tissues and is no longer expressed in normal tissues with development, so it is used as an immunotherapy target (Figure 2).
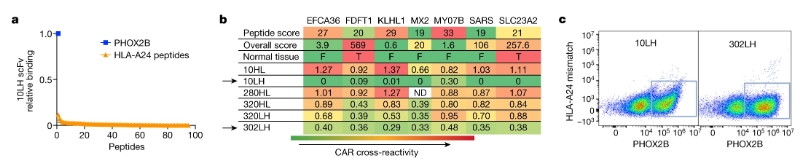
Due to the lack of immunogenicity of self-antigens, after no high-affinity TCR was found in multiple screenings, researchers chose to develop scFv-based CARs, namely peptide-centric chimeric antigen receptors (PC-CARs). First, scFvs that can bind to pMHC were selected by flow cytometry, and clones showing obvious selectivity were further tested. Finally, two scFvs with the strongest specific binding to antigen peptides were screened: 10LH and 302LH (Figure 3), and peptide-centric chimeric antigen receptors T cells (PC-CAR T) were developed.
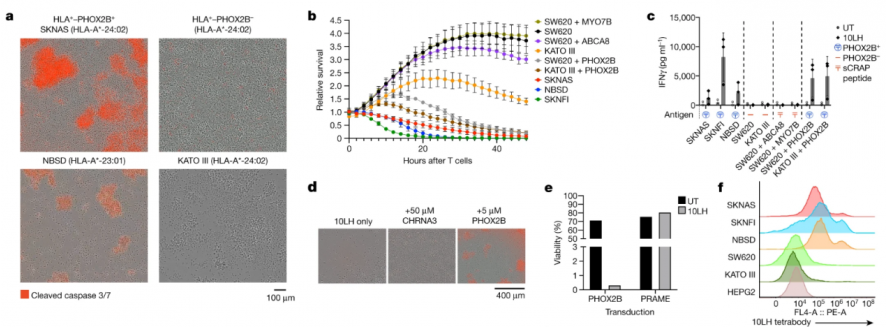
The researchers demonstrated the powerful tumor-specific killing ability of PC-CAR T in in vivo and in vitro experiments. The researchers first tested the potent and specific killing effect of PC-CAR T in neuroblastoma cell lines (SKNAS, NBSD and SKNFI) (Figure 4), and in three cell lines that do not express PHOX2B (SW620, colorectal The functional cross-reactivity of PC-CAR T with the peptide environment presented by off-target tissues was tested in adenocarcinoma cell line; KATO III, gastric adenocarcinoma cell line; and HEPG2, hepatocellular carcinoma cell line) (Figure 3) and the results showed that PC- CAR T cells can selectively eliminate tumors without targeting and killing PHOX2B-negative cell lines.
At the same time, the researchers also used PC-CAR T to treat mice transplanted with neuroblastoma allograft tumors, and found that the tumors were completely eliminated, reaching the treatment endpoint (Figure 5); and this was consistent with the use of untreated CAR T cells. The endpoint tumors showed a significant upregulation of PC-CAR T cell infiltration and MHC expression, suggesting that the therapy can activate T cell expansion at low antigen density, initiating a feedforward cascade that increases MHC and antigen presentation.

These data indicate that PC-CARs have the potential to expand the scope of immunotherapy targets, with the focus and difficulty being in finding antigenic peptides and screening specifically binding scFvs. This strategy would encompass non-immunogenic intracellular tumor proteins and could be therapeutically targeted in a clinical setting by targeting additional HLA allotypes. PC-CARs have several distinct advantages over TCRs in targeting MHC-presented antigenic peptides: PC-CARs can target essential unmutated oncoproteins by inducing immunogenicity using synthetic PC receptors, This opens up the possibility of achieving a higher degree of peptide specificity and targeting through multiple HLAs; in addition, PC-CARs may also have precursors to initiate MHC upregulation and increased antigen density compared to CARs targeting membrane proteins. The ability to feed cascade reactions.
The method introduced in this study will help discover specific targets in other cancer types, and is expected to construct a scFv-based synthetic immunotherapy library to provide a wider HLA genotype coverage population for neoantigens and autoantigens, and is expected to expand The scope of application of cellular immunotherapy such as CAR T.
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies and let more people know about the new development of biomedicine. The content of this article is only used for information exchange. This platform is neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information of this article shall not be used for diagnosis or treatment, can not substitute for professional medical opinion, the company website will not bear any responsibility. The final interpretation of the above statement belongs to the company website, this statement will apply to all the time shared articles, thank you for your cooperation! Copyright: The copyright of the article belongs to Shenzhen Cell Valley, and individuals are welcome to forward it to the circle of friends. Media or institutions, who will reprint it to other platforms in any form without authorization, will be regarded as infringement. If you need to reprint, please contact the email address: contact@sz-cell.com

Researchers including John M. Maris of the University of Pennsylvania School of Medicine and Mark Yarmarkovich of the New York University School of Medicine first used mass spectrometry to analyze neuroblastoma (eight types of neuroblastoma) by performing MHC capture, peptide elution, and liquid chromatography and tandem mass spectrometry. (tumor cell-derived xenografts and patient-derived xenografts), and then compared the immune peptide group presented by MHC I and the immune peptide group potentially presented by normal tissue cells to screen for peptides specifically presented by the tumor, and finally determined the differences. Antigenic peptide PHOX2B. PHOX2B is only expressed in tumor tissues and is no longer expressed in normal tissues with development, so it is used as an immunotherapy target (Figure 2).

Due to the lack of immunogenicity of self-antigens, after no high-affinity TCR was found in multiple screenings, researchers chose to develop scFv-based CARs, namely peptide-centric chimeric antigen receptors (PC-CARs). First, scFvs that can bind to pMHC were selected by flow cytometry, and clones showing obvious selectivity were further tested. Finally, two scFvs with the strongest specific binding to antigen peptides were screened: 10LH and 302LH (Figure 3), and peptide-centric chimeric antigen receptors T cells (PC-CAR T) were developed.

The researchers demonstrated the powerful tumor-specific killing ability of PC-CAR T in in vivo and in vitro experiments. The researchers first tested the potent and specific killing effect of PC-CAR T in neuroblastoma cell lines (SKNAS, NBSD and SKNFI) (Figure 4), and in three cell lines that do not express PHOX2B (SW620, colorectal The functional cross-reactivity of PC-CAR T with the peptide environment presented by off-target tissues was tested in adenocarcinoma cell line; KATO III, gastric adenocarcinoma cell line; and HEPG2, hepatocellular carcinoma cell line) (Figure 3) and the results showed that PC- CAR T cells can selectively eliminate tumors without targeting and killing PHOX2B-negative cell lines.

At the same time, the researchers also used PC-CAR T to treat mice transplanted with neuroblastoma allograft tumors, and found that the tumors were completely eliminated, reaching the treatment endpoint (Figure 5); and this was consistent with the use of untreated CAR T cells. The endpoint tumors showed a significant upregulation of PC-CAR T cell infiltration and MHC expression, suggesting that the therapy can activate T cell expansion at low antigen density, initiating a feedforward cascade that increases MHC and antigen presentation.

These data indicate that PC-CARs have the potential to expand the scope of immunotherapy targets, with the focus and difficulty being in finding antigenic peptides and screening specifically binding scFvs. This strategy would encompass non-immunogenic intracellular tumor proteins and could be therapeutically targeted in a clinical setting by targeting additional HLA allotypes. PC-CARs have several distinct advantages over TCRs in targeting MHC-presented antigenic peptides: PC-CARs can target essential unmutated oncoproteins by inducing immunogenicity using synthetic PC receptors, This opens up the possibility of achieving a higher degree of peptide specificity and targeting through multiple HLAs; in addition, PC-CARs may also have precursors to initiate MHC upregulation and increased antigen density compared to CARs targeting membrane proteins. The ability to feed cascade reactions.
The method introduced in this study will help discover specific targets in other cancer types, and is expected to construct a scFv-based synthetic immunotherapy library to provide a wider HLA genotype coverage population for neoantigens and autoantigens, and is expected to expand The scope of application of cellular immunotherapy such as CAR T.
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies and let more people know about the new development of biomedicine. The content of this article is only used for information exchange. This platform is neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information of this article shall not be used for diagnosis or treatment, can not substitute for professional medical opinion, the company website will not bear any responsibility. The final interpretation of the above statement belongs to the company website, this statement will apply to all the time shared articles, thank you for your cooperation! Copyright: The copyright of the article belongs to Shenzhen Cell Valley, and individuals are welcome to forward it to the circle of friends. Media or institutions, who will reprint it to other platforms in any form without authorization, will be regarded as infringement. If you need to reprint, please contact the email address: contact@sz-cell.com



