CRISPR/CAS9 technology in the field of cell therapy
1、Introduction to CRISPR/Cas system
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeat), first discovered in the late 1980s, is an unusual genetic structure composed of alternating repeating and non-repeating DNA sequences. The CRISPR/Cas system is essentially a bacterial defense mechanism against foreign DNA. Genomic analysis has shown that CRISPR and Cas proteins function as an adaptive immune system and protect prokaryotic DNA from phage and plasmid DNA attacks through an RNA-guided DNA cleavage system [1, 2].
There are many types of CRISPR/Cas systems, among which the CRISPR/Cas9 system is currently the most in-depth research, the most technically mature, and the most widely used type. The CRISPR/Cas9 system consists of a single-stranded guide RNA (sgRNA) and a Cas9 protein with endonuclease function. Under the specific recognition of sgRNA, the Cas9 protein reaches a specific site in the genome, cuts double-stranded DNA (dsDNA), and generates a dsDNA break (double-strand break, DSB). Then, the DSB is repaired through cell-autonomous nonhomologous DNA end joining (NHEJ) or homologous recombination (homology-directed repair (HDR)), of which the dominant NHEJ repair easily causes mutations [3].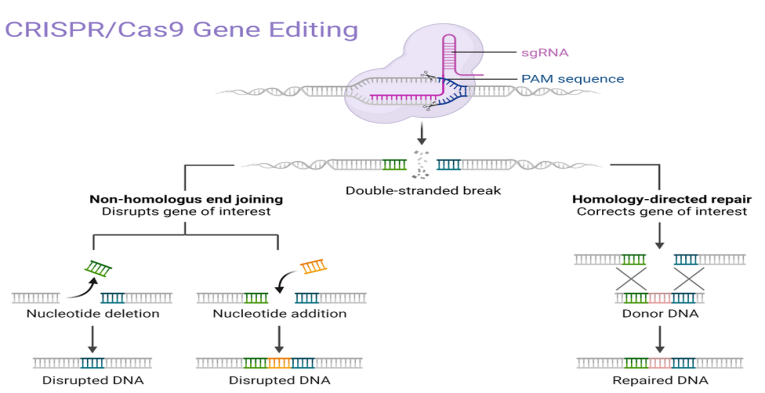
Figure 1. How the CRISPR/Cas9 system works (image from biorender)
2、CRISPR/Cas9 system delivery technology
Natural Cas proteins still have many limitations in terms of editing efficiency and specificity, and may cleave DNA double strands at misplaced gene sites, resulting in potential risks and significantly affecting its practical application. Researchers have developed different Cas9 variants to reduce the mismatch rate and increase editing efficiency [4-6].
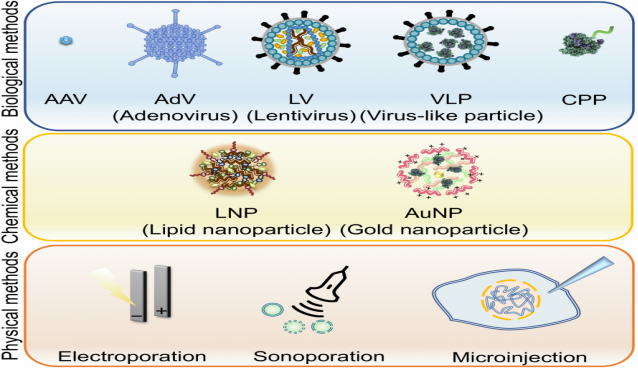
The delivery method of the CRISPR/Cas9 system is also one of the key factors affecting the efficiency of gene editing [7]. Delivery mainly takes three forms: ① Delivery of plasmid DNA encoding Cas9 and sgRNA; ② Delivery of Cas9 mRNA and sgRNA elements; ③ Delivery of RNP (ribonucleoprotein, Cas9 protein and sgRNA complex). Different delivery methods are selected according to different delivery forms, which can be divided into three main groups according to the cell entry mechanism: (1) biological methods, (2) chemical methods, and (3) physical methods. Biological methods utilize natural biological materials such as viral proteins, peptides or cellular receptors/membranes to mediate entry into cells. This category includes viral vectors, virus-like particles (VLPs), and cell-penetrating peptides (CPPs). Chemical methods use synthetic materials, such as polymers, lipids, or metals, to facilitate entry into cells. This category includes liposomes, gold nanoparticles (AuNPs), and lipid nanoparticles (LNPs). Physical methods rely on the physical energy of electricity or ultrasound to deliver genes into cells. This category includes electroporation, sonoporation, and microinjection.
3、Research progress of CRISPR/Cas9 technology in gene and cell therapy
Compared with first- and second-generation gene editing technologies, CRISPR/Cas9 technology has excellent performance in gene knockout, gene silencing, gene knock-in, gene activation and epigenetic modification due to its convenient, simple and efficient operation. Widely used in biomedical research. A report titled "CRISPR and Cas Gene Market - Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2018-2026" released by Transparency Market Research (TMR) stated that by 2026, the global CRISPR and Cas gene market will be The market value reached US$7.2345 billion, with a compound annual growth rate of approximately 20.1% from 2018 to 2026. Gene editing technology for prevention of various diseases, rising demand for alternative medicine for chronic diseases and increasing investments by key players in Asia Pacific are expected to be the major factors driving the market from 2018 to 2026.
(1) CRISPR/Cas9 genome-wide functional screening
By constructing CRISPR-Cas9 libraries, some drug targets and drug-resistant genes can be screened, analyzed, and verified on a large scale to provide relevant data for disease treatment. Researchers used the CRISPR-Cas9 library to screen potential therapeutic targets in human gastric cancer AGS cells across the genome, and finally 41 essential genes were identified as potential drug targets [8]. In addition, the CRISPR-Cas9 library was used to screen 685 gene sites of enhancer elements of the key transcription factors p53 and ERα in cancer cells, and a total of 3 enhancer elements related to ERα were identified; 3 enhancer elements were related to p53 Functionally related, two of the enhancing elements are fully combined with p53 to activate the cellular aging process [9].
(2) Model construction
Disease models are mainly used to simulate the biological and pathological characteristics of human diseases, and play a key role in the pathogenesis of diseases and drug screening.
Most animal models are constructed by knocking out specific genes [10]. Using CRISPR technology to target exon 3 of the monkey SHANK21 gene, researchers successfully constructed an ASD model of the monkey and its F1 offspring due to SHANK3 gene mutation, showing atypical autism phenotypes such as increased repetitive behaviors and social and learning deficits [11]. Point mutation knockin based on single base editing has great potential in building animal models. The researchers ended gene expression early by making single-base edits to stop codons at RAG1, RAG2, and IL2RG sites. After these embryos were implanted into surrogate pigs, it was found that piglets carrying homozygous or heterozygous mutations would suffer from severe underdevelopment of the thymus and spleen, resulting in immune deficiency and lung infection. It was also the first time that single-base editing techniques had been used in large animals [12]. In addition, the construction of cell models in vitro also helps to screen drug action targets and provide basis for revealing drug action mechanisms and disease mechanisms.
(3) Cell therapy
Immunotherapy is a method of killing tumor cells by mobilizing the patient's own immune system, which is mainly divided into immune checkpoint blocking and chimeric antigen receptor T-cell immunotherapy (CAR-T). CRISPR/Cas9 technology is different from the random integration of transgenic technology, which can accurately target target genes and carry out genetic modification. Due to the presence of endogenous HLA and TCR in allogeneic CAR-T cells, which hinder the wide application of therapy, multiple therapeutic strategies have been proposed to prepare CAR-T. Consider the important role of granulocyte-macrophage colony-stimulating factor (GM-CSF) in inducing cytokine storms. In 2019, Sterner et al. [13] used CRISPR/Cas9 to knock out the GM-CSF gene of CD19 CAR-T cells. The results of this study showed that GM-CSF secretion of CAR T cells with GM-CSF gene knockout was indeed significantly reduced, but the important function of CAR T cells was not affected. More importantly, GM-CSF-deficient CD19 CAR T cells showed significant anti-tumor effects in vivo compared with wild-type CD19 CAR T cells. In 2020, Carl June et al. [14] removed endogenous T cell receptor (TCR) and immune checkpoint molecule programmed cell death protein 1 (PD-1) to improve the function and persistence of engineered T cells. A synthetic cancer-specific TCR transgene (NY-ESO-1) was introduced to identify tumor cells (the first multiple CRISPR/Cas9 editing engineering applied to clinical trials). In the same year, the results of the first human phase I clinical trial of CRISPR-Cas9-edited PD-1 T cells in patients with advanced non-small cell lung cancer (ClinicalTrials.gov NCT02793856) were obtained by Professor Lu Youu of China, and the remaining clinical trials were shown in the following table [15].

(4) Gene therapy
Gene therapy refers to the introduction of exogenous normal genes into target cells to correct or compensate for diseases caused by defective and abnormal genes to achieve therapeutic purposes. CRISPR/Cas9 technology has been used for gene therapy of tumors, neurodegenerative diseases, hematopoietic diseases, AIDS and other diseases due to its high editing efficiency and ability to target multiple target genes simultaneously.
CRISPR Therapeutics announced early success with its therapy called CTX001, which delivers CRISPR-Cas9 to hematopoietic stem cells via electroporation. After the patient's own hematopoietic stem cells are chemically eliminated with busulfan, the hematopoietic stem cells edited by CRISPR-Cas9 are infused back into the patient. Treated patients with β-thalassemia or sickle cell disease no longer rely on blood transfusions for long periods of time [16].
NTLA-2001 is being developed as a single-dose treatment for transthyretin (ATTR) amyloidosis. NTLA-2001 is an in vivo gene editing therapeutic designed to treat ATTR amyloidosis by reducing the concentration of TTR in serum. This therapy is based on CRISPR/Cas, and it is the first CRISPR therapy to be delivered systemically in the form of lipid nanoparticles into the blood [17].
EDIT-101, a product developed by Editas Medicine that is still in clinical trials. The drug uses AAV-mediated delivery of CRISPR/Cas9 through sgRNA targeting to remove the abnormal splice donor produced by the IVS290 mutation in the CEP26 gene and restore normal CEP290 expression to treat congenital amaurosis. This therapy can be cured with one administration (NCT03872479).
Reference:
[1] RAN F A, HSU P D, WRIGHT J, et al. Genome engineering using the CRISPR-Cas9 system [J]. Nat Protoc, 2013, 8(11): 2281-308.
[2] MEI Y, WANG Y, CHEN H, et al. Recent Progress in CRISPR/Cas9 Technology [J]. J Genet Genomics, 2016, 43(2): 63-75.
[3] LI T, YANG Y, QI H, et al. CRISPR/Cas9 therapeutics: progress and prospects [J]. Signal Transduct Target Ther, 2023, 8(1): 36.
[4] KLEINSTIVER B P, PATTANAYAK V, PREW M S, et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects [J]. Nature, 2016, 529(7587): 490-5.
[5] CHEN J S, DAGDAS Y S, KLEINSTIVER B P, et al. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy [J]. Nature, 2017, 550(7676): 407-10.
[6] SLAYMAKER I M, GAO L, ZETSCHE B, et al. Rationally engineered Cas9 nucleases with improved specificity [J]. Science, 2016, 351(6268): 84-8.
[7] TAHA E A, LEE J, HOTTA A. Delivery of CRISPR-Cas tools for in vivo genome editing therapy: Trends and challenges [J]. J Control Release, 2022, 342: 345-61.
[8] ZENG Z, ZHANG X, JIANG C Q, et al. Identifying novel therapeutic targets in gastric cancer using genome-wide CRISPR-Cas9 screening [J]. Oncogene, 2022, 41(14): 2069-78.
[9] KORKMAZ G, LOPES R, UGALDE A P, et al. Functional genetic screens for enhancer elements in the human genome using CRISPR-Cas9 [J]. Nat Biotechnol, 2016, 34(2): 192-8. (in Chinese)
[10] TORRES-RUIZ R, RODRIGUEZ-PERALES S. CRISPR-Cas9 technology: applications and human disease modelling [J]. Brief Funct Genomics, 2017, 16(1): 4-12.
[11] ZHOU Y, SHARMA J, KE Q, et al. Atypical behaviour and connectivity in SHANK3-mutant macaques [J]. Nature, 2019, 570(7761): 326-31.
[12] XIE J, GE W, LI N, et al. Efficient base editing for multiple genes and loci in pigs using base editors [J]. Nat Commun, 2019, 10(1): 2852.
[13] STERNER R M, COX M J, SAKEMURA R, et al. Using CRISPR/Cas9 to Knock Out GM-CSF in CAR-T Cells [J]. J Vis Exp, 2019, (149).
[14] STADTMAUER E A, FRAIETTA J A, DAVIS M M, et al. CRISPR-engineered T cells in patients with refractory cancer [J]. Science, 2020, 367(6481).
[15] KATTI A, DIAZ B J, CARAGINE C M, et al. CRISPR in cancer biology and therapy [J]. Nat Rev Cancer, 2022, 22(5): 259-79.
[16] FRANGOUL H, ALTSHULER D, CAPPELLINI M D, et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and beta-Thalassemia [J]. N Engl J Med, 2021, 384(3): 252-60.
[17] LEDFORD H. CRISPR treatment inserted directly into the body for first time [J]. Nature, 2020, 579(7798): 185.
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies and let more people understand the new developments in biomedicine.The content of this article is for information exchange only. This platform remains neutral with respect to the content, statements, and opinion judgments in the article, and does not represent the position and opinions of Shenzhen Cell Valley.The relevant information in this article should not be used for diagnosis or treatment, and cannot replace professional medical advice. Our website will not assume any responsibility.The final interpretation of the above statement belongs to our company’s website. This statement will apply to articles shared on our website at all times. Thank you for your cooperation! Copyright statement: The copyright of the article belongs to Shenzhen Cell Valley. Individuals are welcome to forward it to friends, media or Any unauthorized reproduction by the organization to other platforms will be regarded as infringement.If you need to reprint, please contact email: contact@sz-cell.com
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeat), first discovered in the late 1980s, is an unusual genetic structure composed of alternating repeating and non-repeating DNA sequences. The CRISPR/Cas system is essentially a bacterial defense mechanism against foreign DNA. Genomic analysis has shown that CRISPR and Cas proteins function as an adaptive immune system and protect prokaryotic DNA from phage and plasmid DNA attacks through an RNA-guided DNA cleavage system [1, 2].
There are many types of CRISPR/Cas systems, among which the CRISPR/Cas9 system is currently the most in-depth research, the most technically mature, and the most widely used type. The CRISPR/Cas9 system consists of a single-stranded guide RNA (sgRNA) and a Cas9 protein with endonuclease function. Under the specific recognition of sgRNA, the Cas9 protein reaches a specific site in the genome, cuts double-stranded DNA (dsDNA), and generates a dsDNA break (double-strand break, DSB). Then, the DSB is repaired through cell-autonomous nonhomologous DNA end joining (NHEJ) or homologous recombination (homology-directed repair (HDR)), of which the dominant NHEJ repair easily causes mutations [3].

Figure 1. How the CRISPR/Cas9 system works (image from biorender)
2、CRISPR/Cas9 system delivery technology
Natural Cas proteins still have many limitations in terms of editing efficiency and specificity, and may cleave DNA double strands at misplaced gene sites, resulting in potential risks and significantly affecting its practical application. Researchers have developed different Cas9 variants to reduce the mismatch rate and increase editing efficiency [4-6].
The delivery method of the CRISPR/Cas9 system is also one of the key factors affecting the efficiency of gene editing [7]. Delivery mainly takes three forms: ① Delivery of plasmid DNA encoding Cas9 and sgRNA; ② Delivery of Cas9 mRNA and sgRNA elements; ③ Delivery of RNP (ribonucleoprotein, Cas9 protein and sgRNA complex). Different delivery methods are selected according to different delivery forms, which can be divided into three main groups according to the cell entry mechanism: (1) biological methods, (2) chemical methods, and (3) physical methods. Biological methods utilize natural biological materials such as viral proteins, peptides or cellular receptors/membranes to mediate entry into cells. This category includes viral vectors, virus-like particles (VLPs), and cell-penetrating peptides (CPPs). Chemical methods use synthetic materials, such as polymers, lipids, or metals, to facilitate entry into cells. This category includes liposomes, gold nanoparticles (AuNPs), and lipid nanoparticles (LNPs). Physical methods rely on the physical energy of electricity or ultrasound to deliver genes into cells. This category includes electroporation, sonoporation, and microinjection.

3、Research progress of CRISPR/Cas9 technology in gene and cell therapy
Compared with first- and second-generation gene editing technologies, CRISPR/Cas9 technology has excellent performance in gene knockout, gene silencing, gene knock-in, gene activation and epigenetic modification due to its convenient, simple and efficient operation. Widely used in biomedical research. A report titled "CRISPR and Cas Gene Market - Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2018-2026" released by Transparency Market Research (TMR) stated that by 2026, the global CRISPR and Cas gene market will be The market value reached US$7.2345 billion, with a compound annual growth rate of approximately 20.1% from 2018 to 2026. Gene editing technology for prevention of various diseases, rising demand for alternative medicine for chronic diseases and increasing investments by key players in Asia Pacific are expected to be the major factors driving the market from 2018 to 2026.
(1) CRISPR/Cas9 genome-wide functional screening
By constructing CRISPR-Cas9 libraries, some drug targets and drug-resistant genes can be screened, analyzed, and verified on a large scale to provide relevant data for disease treatment. Researchers used the CRISPR-Cas9 library to screen potential therapeutic targets in human gastric cancer AGS cells across the genome, and finally 41 essential genes were identified as potential drug targets [8]. In addition, the CRISPR-Cas9 library was used to screen 685 gene sites of enhancer elements of the key transcription factors p53 and ERα in cancer cells, and a total of 3 enhancer elements related to ERα were identified; 3 enhancer elements were related to p53 Functionally related, two of the enhancing elements are fully combined with p53 to activate the cellular aging process [9].
(2) Model construction
Disease models are mainly used to simulate the biological and pathological characteristics of human diseases, and play a key role in the pathogenesis of diseases and drug screening.
Most animal models are constructed by knocking out specific genes [10]. Using CRISPR technology to target exon 3 of the monkey SHANK21 gene, researchers successfully constructed an ASD model of the monkey and its F1 offspring due to SHANK3 gene mutation, showing atypical autism phenotypes such as increased repetitive behaviors and social and learning deficits [11]. Point mutation knockin based on single base editing has great potential in building animal models. The researchers ended gene expression early by making single-base edits to stop codons at RAG1, RAG2, and IL2RG sites. After these embryos were implanted into surrogate pigs, it was found that piglets carrying homozygous or heterozygous mutations would suffer from severe underdevelopment of the thymus and spleen, resulting in immune deficiency and lung infection. It was also the first time that single-base editing techniques had been used in large animals [12]. In addition, the construction of cell models in vitro also helps to screen drug action targets and provide basis for revealing drug action mechanisms and disease mechanisms.
(3) Cell therapy
Immunotherapy is a method of killing tumor cells by mobilizing the patient's own immune system, which is mainly divided into immune checkpoint blocking and chimeric antigen receptor T-cell immunotherapy (CAR-T). CRISPR/Cas9 technology is different from the random integration of transgenic technology, which can accurately target target genes and carry out genetic modification. Due to the presence of endogenous HLA and TCR in allogeneic CAR-T cells, which hinder the wide application of therapy, multiple therapeutic strategies have been proposed to prepare CAR-T. Consider the important role of granulocyte-macrophage colony-stimulating factor (GM-CSF) in inducing cytokine storms. In 2019, Sterner et al. [13] used CRISPR/Cas9 to knock out the GM-CSF gene of CD19 CAR-T cells. The results of this study showed that GM-CSF secretion of CAR T cells with GM-CSF gene knockout was indeed significantly reduced, but the important function of CAR T cells was not affected. More importantly, GM-CSF-deficient CD19 CAR T cells showed significant anti-tumor effects in vivo compared with wild-type CD19 CAR T cells. In 2020, Carl June et al. [14] removed endogenous T cell receptor (TCR) and immune checkpoint molecule programmed cell death protein 1 (PD-1) to improve the function and persistence of engineered T cells. A synthetic cancer-specific TCR transgene (NY-ESO-1) was introduced to identify tumor cells (the first multiple CRISPR/Cas9 editing engineering applied to clinical trials). In the same year, the results of the first human phase I clinical trial of CRISPR-Cas9-edited PD-1 T cells in patients with advanced non-small cell lung cancer (ClinicalTrials.gov NCT02793856) were obtained by Professor Lu Youu of China, and the remaining clinical trials were shown in the following table [15].
Table 1 Ongoing clinical trials of immunotherapies designed using CRISPR technology to treat human cancer

(4) Gene therapy
Gene therapy refers to the introduction of exogenous normal genes into target cells to correct or compensate for diseases caused by defective and abnormal genes to achieve therapeutic purposes. CRISPR/Cas9 technology has been used for gene therapy of tumors, neurodegenerative diseases, hematopoietic diseases, AIDS and other diseases due to its high editing efficiency and ability to target multiple target genes simultaneously.
CRISPR Therapeutics announced early success with its therapy called CTX001, which delivers CRISPR-Cas9 to hematopoietic stem cells via electroporation. After the patient's own hematopoietic stem cells are chemically eliminated with busulfan, the hematopoietic stem cells edited by CRISPR-Cas9 are infused back into the patient. Treated patients with β-thalassemia or sickle cell disease no longer rely on blood transfusions for long periods of time [16].
NTLA-2001 is being developed as a single-dose treatment for transthyretin (ATTR) amyloidosis. NTLA-2001 is an in vivo gene editing therapeutic designed to treat ATTR amyloidosis by reducing the concentration of TTR in serum. This therapy is based on CRISPR/Cas, and it is the first CRISPR therapy to be delivered systemically in the form of lipid nanoparticles into the blood [17].
EDIT-101, a product developed by Editas Medicine that is still in clinical trials. The drug uses AAV-mediated delivery of CRISPR/Cas9 through sgRNA targeting to remove the abnormal splice donor produced by the IVS290 mutation in the CEP26 gene and restore normal CEP290 expression to treat congenital amaurosis. This therapy can be cured with one administration (NCT03872479).
Reference:
[1] RAN F A, HSU P D, WRIGHT J, et al. Genome engineering using the CRISPR-Cas9 system [J]. Nat Protoc, 2013, 8(11): 2281-308.
[2] MEI Y, WANG Y, CHEN H, et al. Recent Progress in CRISPR/Cas9 Technology [J]. J Genet Genomics, 2016, 43(2): 63-75.
[3] LI T, YANG Y, QI H, et al. CRISPR/Cas9 therapeutics: progress and prospects [J]. Signal Transduct Target Ther, 2023, 8(1): 36.
[4] KLEINSTIVER B P, PATTANAYAK V, PREW M S, et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects [J]. Nature, 2016, 529(7587): 490-5.
[5] CHEN J S, DAGDAS Y S, KLEINSTIVER B P, et al. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy [J]. Nature, 2017, 550(7676): 407-10.
[6] SLAYMAKER I M, GAO L, ZETSCHE B, et al. Rationally engineered Cas9 nucleases with improved specificity [J]. Science, 2016, 351(6268): 84-8.
[7] TAHA E A, LEE J, HOTTA A. Delivery of CRISPR-Cas tools for in vivo genome editing therapy: Trends and challenges [J]. J Control Release, 2022, 342: 345-61.
[8] ZENG Z, ZHANG X, JIANG C Q, et al. Identifying novel therapeutic targets in gastric cancer using genome-wide CRISPR-Cas9 screening [J]. Oncogene, 2022, 41(14): 2069-78.
[9] KORKMAZ G, LOPES R, UGALDE A P, et al. Functional genetic screens for enhancer elements in the human genome using CRISPR-Cas9 [J]. Nat Biotechnol, 2016, 34(2): 192-8. (in Chinese)
[10] TORRES-RUIZ R, RODRIGUEZ-PERALES S. CRISPR-Cas9 technology: applications and human disease modelling [J]. Brief Funct Genomics, 2017, 16(1): 4-12.
[11] ZHOU Y, SHARMA J, KE Q, et al. Atypical behaviour and connectivity in SHANK3-mutant macaques [J]. Nature, 2019, 570(7761): 326-31.
[12] XIE J, GE W, LI N, et al. Efficient base editing for multiple genes and loci in pigs using base editors [J]. Nat Commun, 2019, 10(1): 2852.
[13] STERNER R M, COX M J, SAKEMURA R, et al. Using CRISPR/Cas9 to Knock Out GM-CSF in CAR-T Cells [J]. J Vis Exp, 2019, (149).
[14] STADTMAUER E A, FRAIETTA J A, DAVIS M M, et al. CRISPR-engineered T cells in patients with refractory cancer [J]. Science, 2020, 367(6481).
[15] KATTI A, DIAZ B J, CARAGINE C M, et al. CRISPR in cancer biology and therapy [J]. Nat Rev Cancer, 2022, 22(5): 259-79.
[16] FRANGOUL H, ALTSHULER D, CAPPELLINI M D, et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and beta-Thalassemia [J]. N Engl J Med, 2021, 384(3): 252-60.
[17] LEDFORD H. CRISPR treatment inserted directly into the body for first time [J]. Nature, 2020, 579(7798): 185.
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies and let more people understand the new developments in biomedicine.The content of this article is for information exchange only. This platform remains neutral with respect to the content, statements, and opinion judgments in the article, and does not represent the position and opinions of Shenzhen Cell Valley.The relevant information in this article should not be used for diagnosis or treatment, and cannot replace professional medical advice. Our website will not assume any responsibility.The final interpretation of the above statement belongs to our company’s website. This statement will apply to articles shared on our website at all times. Thank you for your cooperation! Copyright statement: The copyright of the article belongs to Shenzhen Cell Valley. Individuals are welcome to forward it to friends, media or Any unauthorized reproduction by the organization to other platforms will be regarded as infringement.If you need to reprint, please contact email: contact@sz-cell.com


