Shenzhen Cell Valley achieved a major breakthrough in CAR-NK preparation process
In response to the pain points in the early R&D and industrial production of CAR-NK cells, Shenzhen Cell Valley's core R&D team conducted long-term research on its underlying technology, and finally made a breakthrough in the CAR-NK cell preparation process: using Shenzhen Cell Valley's unique The retroviral vector produced by the PackRV-SS system can transduce NK cells. The transduction efficiency is stable at more than 60%, and the positive rate has remained stable; and CAR-NK cells can proliferate up to 30 days after transduction. 10,000 times. This breakthrough development will greatly promote the product development and clinical application of CAR-NK cells. Enterprises and institutions engaged in NK and CAR-NK cell therapy are welcome to cooperate with Shenzhen Cell Valley to jointly promote the development of the industry.
In the past two years, in addition to CAR-T therapy that has attracted much attention, another new immune cell therapy - natural killer (NK) cell therapy is also regarded as a new generation of anti-tumor weapon for the special treatment of solid tumors. However, the main problems currently faced by CAR-NK production include: low and unstable exogenous gene transduction efficiency; difficulty in culturing NK cells and low in vitro proliferation multiples, which cannot meet the cell dosage for clinical treatment; large initial viral vector dosage and high cost Gao Gao etc. Retroviruses using new envelope designs can achieve efficient transduction of primary NK cells and achieve stable, economical, and large-scale production of retroviral vectors through stable toxin-producing monoclonal packaging cell lines.

The core R&D team of Shenzhen Cell Valley used a new envelope-designed retroviral vector to transduce NK cells, and the CAR-NK positive rate was 67.7% 2 days after viral transduction. And the positive rate of CAR-NK was continuously tested until the 27th day after viral transduction. The positive rate of CAR-NK was still maintained at 71%, which was at a stable and high transduction level. At the same time, 10 days after transduction, the purity of NK cells (CD56+CD3-) in CAR-NK cells was always maintained above 95%, and the content of T cells (CD56-CD3+) was less than 0.5%. The purity is extremely high and has reached and far exceeded the allogeneic transplant standards recognized by the US FDA (NK cells are greater than 90%, T cells are less than 5%), and can avoid the occurrence of GVHD.
2、The proliferation of CAR-NK exceeds 10,000 times, which can meet the cell dosage requirements for non-clinical experiments and even clinical trials.
The research team continuously monitored the proliferation status of untransduced NK cells and transduced CAR-NK cells. NK cells multiplied to 15,000-fold after 32 days of culture, and CAR-NK cells multiplied to 10,000-fold after 27 days of culture. Whether it is the proliferation of NK or CAR-NK, they can meet the cell dosage of NK cell therapy on the market. And during continuous expansion, NK cell purity is always greater than 96%. The CAR positive rate in CAR-NK is always greater than 65% within one month, overcoming the problem of declining CAR positive rates reported by other NK cell research institutions.
3、The in vitro killing effect of CAR-NK cells is better than that of NK cells at low-efficiency target ratios (1:1 and 1:3)
In the past two years, in addition to CAR-T therapy that has attracted much attention, another new immune cell therapy - natural killer (NK) cell therapy is also regarded as a new generation of anti-tumor weapon for the special treatment of solid tumors. However, the main problems currently faced by CAR-NK production include: low and unstable exogenous gene transduction efficiency; difficulty in culturing NK cells and low in vitro proliferation multiples, which cannot meet the cell dosage for clinical treatment; large initial viral vector dosage and high cost Gao Gao etc. Retroviruses using new envelope designs can achieve efficient transduction of primary NK cells and achieve stable, economical, and large-scale production of retroviral vectors through stable toxin-producing monoclonal packaging cell lines.
1、The single-transduction positive rate of CAR-NK reaches more than 60% and remains at a relatively stable level for a long time. There is no decrease in the CAR positive rate.

The core R&D team of Shenzhen Cell Valley used a new envelope-designed retroviral vector to transduce NK cells, and the CAR-NK positive rate was 67.7% 2 days after viral transduction. And the positive rate of CAR-NK was continuously tested until the 27th day after viral transduction. The positive rate of CAR-NK was still maintained at 71%, which was at a stable and high transduction level. At the same time, 10 days after transduction, the purity of NK cells (CD56+CD3-) in CAR-NK cells was always maintained above 95%, and the content of T cells (CD56-CD3+) was less than 0.5%. The purity is extremely high and has reached and far exceeded the allogeneic transplant standards recognized by the US FDA (NK cells are greater than 90%, T cells are less than 5%), and can avoid the occurrence of GVHD.
2、The proliferation of CAR-NK exceeds 10,000 times, which can meet the cell dosage requirements for non-clinical experiments and even clinical trials.
The research team continuously monitored the proliferation status of untransduced NK cells and transduced CAR-NK cells. NK cells multiplied to 15,000-fold after 32 days of culture, and CAR-NK cells multiplied to 10,000-fold after 27 days of culture. Whether it is the proliferation of NK or CAR-NK, they can meet the cell dosage of NK cell therapy on the market. And during continuous expansion, NK cell purity is always greater than 96%. The CAR positive rate in CAR-NK is always greater than 65% within one month, overcoming the problem of declining CAR positive rates reported by other NK cell research institutions.
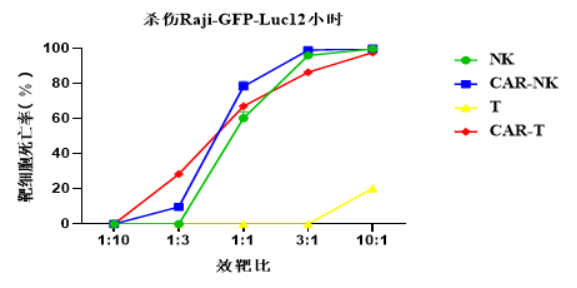
3、The in vitro killing effect of CAR-NK cells is better than that of NK cells at low-efficiency target ratios (1:1 and 1:3)

The research team also conducted a preliminary uation of the in vitro killing capabilities of NK and CAR-NK cells. Experimental results show that at high-efficiency target ratios (3:1 and 10:1), there is no significant difference in the killing effects of NK and CAR-NK, and both have a killing efficiency of more than 90% on target cells. At low-efficiency target ratios (1:3 and 1:1), the killing effect of CAR-NK is better than that of NK cells. Compared with CAR-T, at most effect-to-target ratios (1:1, 3:1 and 10:1), the killing effect of CAR-NK is better than that of CAR-T cells. This data shows that retroviral vectors can enhance the in vitro killing ability of NK cells to a certain extent after transducing NK.
Shenzhen Cell Valley has designed and produced CAR-specific retroviral vector spot products for a variety of tumor targets such as BCMA, CD19, CD38, CD22, Trop2, FRα, CD7, CD33, EGFR/EGFRVIII, etc., and all have achieved The efficient transduction of human primary T cells will also be further studied in the field of immune cell therapy such as NK cells, γδ T cells, and macrophages. Shenzhen Cell Valley warmly welcomes enterprises and institutions in the field of CGT to come for exchanges and guidance. We will take advantage of the huge advantages of retroviral vectors in scientific research and industrial production to jointly develop cell products based on retroviral vectors and jointly promote new CAR-NK cell products. The advent of.
【Currently, Shenzhen Cell Valley can provide trial packs of CD19-CAR and GFP-Luciferase virus products prepared based on retroviral vectors for testing. You can contact the marketing and sales departments at any time to obtain trial pack products.】
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies, so that more people understand the new development of biomedicine. The content of this article is only used for information exchange, and the platform remains neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information in this article should not be used as a diagnosis or treatment, is not a substitute for professional medical advice, and the company's website will not assume any responsibility. The final interpretation of the content of the above statement belongs to the company's website, this statement will apply to the company's website all the time to share the article, thank you for your cooperation! Copyright description: The copyright of the article belongs to Shenzhen Cell Valley, individuals are welcome to forward to the circle of friends, media or institutions without authorization, reproduced in any form to other platforms, will be regarded as infringement. For reprinting, please contact email: contact@sz-cell.com
Shenzhen Cell Valley has designed and produced CAR-specific retroviral vector spot products for a variety of tumor targets such as BCMA, CD19, CD38, CD22, Trop2, FRα, CD7, CD33, EGFR/EGFRVIII, etc., and all have achieved The efficient transduction of human primary T cells will also be further studied in the field of immune cell therapy such as NK cells, γδ T cells, and macrophages. Shenzhen Cell Valley warmly welcomes enterprises and institutions in the field of CGT to come for exchanges and guidance. We will take advantage of the huge advantages of retroviral vectors in scientific research and industrial production to jointly develop cell products based on retroviral vectors and jointly promote new CAR-NK cell products. The advent of.
【Currently, Shenzhen Cell Valley can provide trial packs of CD19-CAR and GFP-Luciferase virus products prepared based on retroviral vectors for testing. You can contact the marketing and sales departments at any time to obtain trial pack products.】
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies, so that more people understand the new development of biomedicine. The content of this article is only used for information exchange, and the platform remains neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information in this article should not be used as a diagnosis or treatment, is not a substitute for professional medical advice, and the company's website will not assume any responsibility. The final interpretation of the content of the above statement belongs to the company's website, this statement will apply to the company's website all the time to share the article, thank you for your cooperation! Copyright description: The copyright of the article belongs to Shenzhen Cell Valley, individuals are welcome to forward to the circle of friends, media or institutions without authorization, reproduced in any form to other platforms, will be regarded as infringement. For reprinting, please contact email: contact@sz-cell.com


